Johnson & Johnson COVID-19 review by FDA external advisory committee
Tagged:COVID
/
PharmaAndBiotech
/
SomebodyAskedMe
/
Statistics
Today the J&J COVID-19 vaccine gets reviewed by VRBPAC (Vaccines & Related Biological Products Advisory Committee) at the FDA, for an EUA (Emergency Use Authorization). Let’s have a look through their submission documents!
What’s the sitch?
An intial amusing confusion: technically, this vaccine was not developed by Johnson & Johnson. It was developed by Janssen Pharmaceuticals, headquartered in Belgium. But in spite of having a separate identity, they were acquired by Johnson & Johnson in 1961. The similar-sounding names lead to no end of amusing confusions.
Now, this crummy little blog is not the first to look into these data, and probably never will be. So we’ll respect the people who have dug into it by examining their conclusions first, and then diving into the FDA submission docs for our own take.
 First up, as usual from STAT News, is an
article by Matthew Herper & Helen Branswell [1]. Their
title (“FDA scientists endorse J&J’s Covid vaccine…”) reminds us of how this
process works. When you submit something to the FDA for approval, something like this happens:
First up, as usual from STAT News, is an
article by Matthew Herper & Helen Branswell [1]. Their
title (“FDA scientists endorse J&J’s Covid vaccine…”) reminds us of how this
process works. When you submit something to the FDA for approval, something like this happens:
- They first throw out your conclusions. Well, not exactly throw out, but at least put away in a drawer where they won’t look at it yet.
- They extract the raw data from the package. They analyze it using exactly the analysis methods specified in the clinical trial protocol as specified at registration.
- Now they get out your conclusions and compare with their own analysis. If they differ, you’re gonna get a frown and a warning letter that you’d better withdraw the application (better to withdraw than to be rejected; it’s hard to resubmit after a rejection).
- If their results match yours, then they are compared with either controls or standard of care to see if there’s reasonable efficacy, and the adverse events are compared with background to see if there’s reasonable safety.
- They make a recommendation to the external review committee.
It’s the output of step 4 & 5 that Herper & Branswell are telling us about. The review committee (VRBPAC) meeting is happening today even as we’re writing this.
The bottom line:
- General picture: This is a single-shot vaccine with a much simpler cold chain requirement than either Moderna or Pfizer. So it’s going to be easier to get into hospitals, clinics, and doctor’s offices. It’s going to be a lot easier to get into the developing world; that’s important because as they say in public health, “none of us is safe until all of us are safe.”
- Vaccine efficacy: There was 66.1% efficacy moderate/severe COVID if you only count infections 28 days after vaccination (which is totally fair). A quick & dirty check: 66 cases in the vaccine arm and 193 in the control arm. If we assume the arms were of exactly the same size – not exactly, but approximately true – then with $N_{vac} = N_{ctl}$ we can get pretty close:
- The odd part, as we reported previously, is that the efficacy numbers are kind of all over the place, depending on where the trial site was! The US site had 72% efficacy, Latin America had 66%, and South Africa had 57%. Which one should we believe? Both the Latin American and South African populations are likely coronavirus variants. South Africa, in particular, has the B.1.351 variant with the N501Y mutation that appears to increase transmission and the E484K mutation that seems to increase escape from immune surveillance. So it’s not surprising that a vaccine based on “coronavirus classic” would be less effective there.
- It’s tempting to compare this unfavorably with the ≥ 95% efficacy of Pfizer/BioNTech and Moderna. But don’t go there: recall those trials were done summer and fall of 2020, when the South African variant ws not circulating (or at least not widely). So the mRNA vaccines have not been tested against these variants. Even now, Pfizer and Moderna are investigating a booster vaccine against the variants and the FDA has promised a streamlined approval process for those boosters, about which more in a later post.
- Vaccine efficacy against severe COVID: They report 81.7% after 28 days, compared to 64% against moderate COVID. So it’s still pretty good at avoiding severe disease.
- Hospitalizations and deaths: If you look 28 days or more past vaccinations, there were 0 COVID hospitalizations or deaths in the treatment arm. There were 16 hospitalizations and 7 deaths in the control arm. That’s 100% efficacy against hospitalization and death. (Confidence intervals later, if we dive deeply enough into the submission docs.)
- Side effects: Mostly the usual. One exception was blood clotting conditions: 15 people in the treatment arm vs 10 in the control arm. So… a little more in the treatment arm, but not much and not very frequently out of 10s of thousands of subjects. Still, the FDA says they’ll “recommend monitoring for thromboembolic events” if an EUA is granted. A bit cautious, but fair.
- Single dose vs double dose: Yes, this trial monitored single dose. But they’re doing a 2-dose trial as well. I think it was Fauci who said he thought the second booster dose would get efficacy up in the 90% range, comparable to Pfizer and Moderna. Interestingly, the Russian Sputnik V/Гам-КОВИД-Вак vaccine also a viral vector vaccine like J&J, and with 2 doses it gets to about 91% efficacy. So there’s a hint of evidence that if you’re willing to give up some convenience of single dose administration, you can get back some higher efficacy. Maybe.

Next up, as now seems to be the custom of this blog for some reason, is the formidable med-chem blogger Derek Lowe at his blog In the Pipeline [2], hosted by Science Translational Medicine. (His blog, by the way, is one of the few Internet venues where the comments are actually worth reading.)
- Lowe confirms a crucial summary fact: this vaccine, like all the others, is observed in randomized clinical trials to prevent completely deaths from COVID-19. So I don’t want to hear any whining in the comments about efficacy differences, ok? Get vaccinated so you’ll live, and not die. Remember that if you remember nothing else here!
- Interestingly, this trial also checked for asymptomatic infection. Unlike the others, which waited until you reported feeling sick, this one took about 2900 people out of the about 10,000 in each arm and tested them regardless of symptoms. The FDA analysis says there’s approximately no protection against asymptomatic infection up to day 29, and about 60% – 70% protection thereafter. This is very important: aysmptomatic carriers are major spreaders of infection, so reducing that is a major step in stopping community transmission.
- Vaccine efficacy: Lowe also points out that efficacy differences between Pfizer/Moderna and J&J are hopelessly convolved with different coronavirus variants in circulation at the time of each trial. If Pfizer/Moderna had to cope with South African B.1.351 variants with the E484K mutation, they might not have looked so great. (Hence, in my view, the need for boosters to cope with the newer variants to get out ahead of the virus evolution!)
- EUA approval is almost certain (and today we’ll find out). Production of an adenovirus vector vaccine is really complicated, as Lowe pointed out in an excellent tutorial on the process. [3] (Of course, making the mRNA vaccines is a bizarre art form of its own, as Lowe also explained. [4]) Anybody who tells you we could “just make more, if only the evil pharma companies would share their designs”… is deeply ignorant and spreading disinformation.
I really like his bottom line:
But the big message is the same: right now, variants and all, we’re winning. The vaccines work, there is a whole list of them, and their production is increasing while we watch. The countries that have gotten off to faster starts vaccinating their populations are already seeing the effects, and no bad safety signals are yet complicating things. Nor are we seeing evidence so far of antibody-dependent enhancement (worse infections recurring in people who have already been vaccinated). If we can keep this pressure up and keep ramping up vaccine supplies and their rollout around the world, we are going to beat this virus. Good riddance to it.
That’s important to understand in the midst of our fear, anxiety, and depression.
The J&J FDA EUA application briefing documents
The briefing documents submitted by Johnson & Johnson (really by Janssen) are up on the FDA web site, along with the FDA internal analysis. [5] [6] [7] [8] [9] [10] As with any such endeavor as important and complex as a drug application, there’s a lot here to troll through! (And we’re not even counting all the clinical trial documents, data reports, safety review board reports, … this is just the summary for the VRBPAC.)
So what’s in there?
Overall claims
 Their summary claims about efficacy, shown here, are:
Their summary claims about efficacy, shown here, are:
- About 85% vaccine efficacy against severe COVID-19, 28 days after injection globally, including the B.1.351 strain in South Africa.
- About 72% vaccine efficacy against moderate-to-severe COVID-19 in the US population and virus strains.
- About 66% vaccine efficacy against moderate-to-severe COVID-19 globally.
- No drastic changes in efficacy across subgroups by age, ethnicity, etc.
When you recall that the FDA’s threshold for “good enough to stop community transmission” is 50% vaccine efficacy, this is clearly good enough. We of course want to see confidence intervals around those numbers, but the summary is good.
The logistical advantages are significant: single dose (though a second dose is being tested), stable for 3 months at ordinary refrigerator temperatures (2° – 8°C), stable for 2 years at ordinary freezer temperatures (-25° – -15°C), large scale manufacturing available (100 million doses in US by 2021H1), and easily shipped by ordinary refrigerator trucks. These are significant advantages, for which we might voluntarily sacrifice a few points of vaccine efficacy. If necessary, we can always come back with Pfizer/Moderna later, so long as we get people immunized now with something like J&J.
Mechanism of action
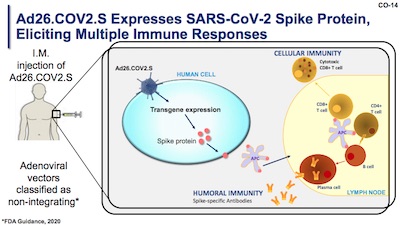 This is a viral vector vaccine. They take an ordinary adenovirus (roughly: “common
cold”), and mess with it in a couple of ways:
This is a viral vector vaccine. They take an ordinary adenovirus (roughly: “common
cold”), and mess with it in a couple of ways:
- They make it replication incompetent, i.e., incapable of growing inside a human body. This is done by genetic engineering on the Ad26 genome so that it can only reproduce in rather bizarre laboratory environments, and so that it has basically no chance to mutate back to a virus capable of infecting humans. So it can enter one cell, one time, no more.
- They add the gene for the SARS-CoV-2 spike protein to the Ad26 genome. So when it enters a cell, it tries to get the cell to make more viral proteins so it can replicate. The Ad26 replication fails, as above. The spike protein gets made, but no other SARS-CoV-2 proteins: it absolutely cannot give you COVID-19.
- The cell in question displays the spike protein on its surface, in essence asking the immune system: “Hey, anybody know if this is supposed to be here?”
- The immune system does something really complicated involving antigen-presenting cells, plasma cells, B cells, T cells of a couple kinds, etc. (And probably more, because… immunology.) The question of whether the spike protein belongs here is answered along the lines of “oh, hell no!”
- Antibody production against the spike protein begins. Memory B cells remember this so they can manufacture more antibodies in the future if needed. Inflammatory cytokines get sprayed all over everything, thereby making you feel like crap for a day. But you are not sick: your immune system thinks you are, and gears up to fight it. But really it’s just being trained in case of a real infection. Think of it as your immune system being sore after a workout at the gym.
The Phase 3 Study Populations
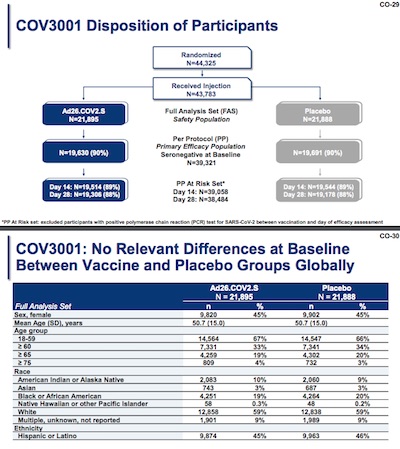
The study design is massive: 44,325 people initially, divide about equally between treatment and control arms. There was an initial safety run-up, to make sure things were ok with a slightly smaller initial group. The interesting places are the primary efficacy population (since day 0, day 14, and day 28). The last population is the most interesting as it gave the subjects’ immune systems time to react; it was the focus in their overall claims. In that case, we’re talking 19,306 treatment and 19,178 controls.
Side note: when vaccine ‘skeptics’ say they think these vaccines haven’t been tested enough, remind them of these numbers! The test populations are huge.
When you break down the trial population by sex, age, race, and so on the result is that the treatment and control groups look pretty similar. This is as it should be. I note with some satisfaction that 23% of the treatment arm subjects were ≥ 65 or ≥ 75 years old. So we have pretty reasonable sampling of response in the elderly.
Vaccine efficacy
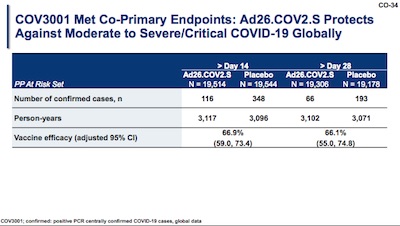
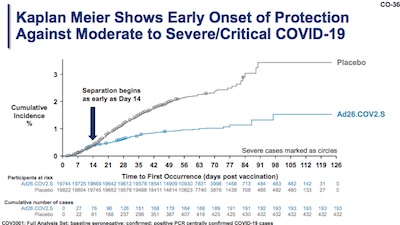 Using the first table, showing results on moderate/severe COVID globally, we can amend our
quick-and-dirty calculation of vaccine efficacy above. Now we know the exact sizes of the
treatment and control arms ($N_{vac}$ and $N_{ctl}$) at day 28, so:
Using the first table, showing results on moderate/severe COVID globally, we can amend our
quick-and-dirty calculation of vaccine efficacy above. Now we know the exact sizes of the
treatment and control arms ($N_{vac}$ and $N_{ctl}$) at day 28, so:
That’s within a gnat’s whisker of the 66.1% result reported here. The remaining difference is probably due to their (proper!) use of Cox regression methods to account for censorship (people dropping out of the trial). We don’t have the censorship data here to do that, but we can get mightily close with just the overall totals.
So they report a vaccine efficacy against moderate/severe COVID-19 of 66.1% with a 95% confidence interval of 55.0% – 74.8%. I haven’t checked the math on the confidence interval, but it looks like they’re using the Clopper-Pearson method (not quite my favorite, but certainly good enough). That means we are 97.5% confident that the true vaccine efficacy is above 55%, which means we beat the 50% efficacy threshold for approval.
The Kaplan-Meier curve (here: percent infections in each arm vs time) is also interesting. It’s not as shockingly beautiful as the one from Pfizer or the one from Moderna, but those were really, wonderfully exceptional. This one is still very good:
- The gray curve, representing the control arm, is rising approximately linearly from day 0 to day 91. (Ok, there’s a little curvature. Doesn’t matter: still rising at a frightening rate.)
- The blue curve, representing the treatment arm, still rises a bit. You aren’t bullet-proof against infection here, but it’s better. In fact, it’s remarkably better than the control arm. Once you get past day 14, or day 28 to be really careful, immunity has set in.
This is evidence the vaccine works.
Efficacy against severe or critical COVID-19
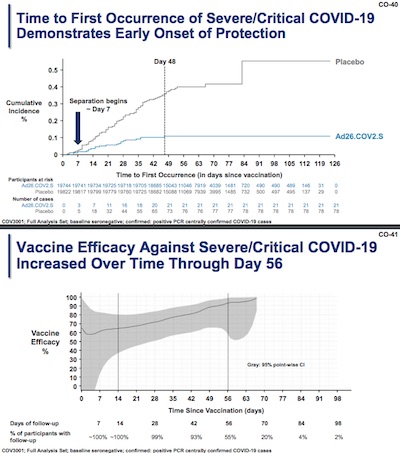 This is where the rubber really hits the road: severe and critical COVID-19 cases lead to
hospitalization and death. That’s what this is all about: relieving the strain on medical
systems by avoiding hospitalization, and people not dying. (I don’t know why we have to
keep repeating that. People somehow lose focus that the goal is not to die.)
This is where the rubber really hits the road: severe and critical COVID-19 cases lead to
hospitalization and death. That’s what this is all about: relieving the strain on medical
systems by avoiding hospitalization, and people not dying. (I don’t know why we have to
keep repeating that. People somehow lose focus that the goal is not to die.)
The Kaplan-Meier curve at the top shows something interesting:
- The blue curve for the treated population is almost completely flat after day 48, meaning that about a month and a half after vaccination the protection against severe/critical COVID is nearly ironclad.
- Also, this protection starts to set in even earlier, at day 7.
This is very good news!
The bottom plot is kind of interesting: it shows the vaccine efficacy calculated apparently with a moving window in time, looking out from day 0 to day 65 or so. The blue curve is (apparently?) the Maximum Likelihood estimator of the vaccine efficacy, i.e., the best bet you can make from the data about efficacy. The gray band around it is a confidence interval, probably 95% confidence (though it doesn’t say exactly). Interesting things to note:
- The confidence interval is broad (uncertain) at the beginning, but narrows as we go along (becoming more certain). (The broadening at the end is probably because the confidence interval depends on future as well as past data in its time window, maybe?)
- The ML estimate rises with time, i.e., the immunity is improving with time from vaccination. Perhaps this is antibody maturation, where the immune system keeps honing its antibodies to be better and better binders to the target spike protein?
This is also very good news!
Group breakdown and viral variants
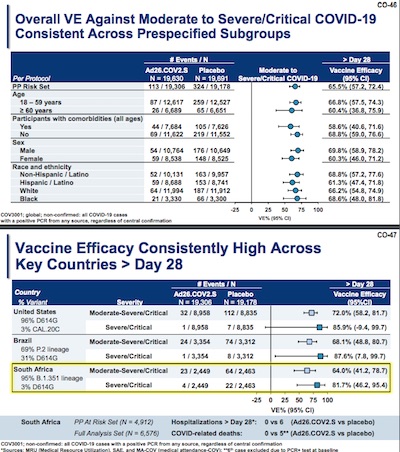 They also broke down the vaccine efficacy by various subject subgroups and by viral
variants. The results look pretty good:
They also broke down the vaccine efficacy by various subject subgroups and by viral
variants. The results look pretty good:
- It’s slightly less efficacious in the ≥ 60 age cohort, though we sort of expect that. In fact, it’s not much less, and the 95% confidence interval is still mostly above 50% (“good enough”).
- The effect on the cohort with comorbidities is no worse than the effect of age.
- Not much difference between men and women (ok, a little; no idea why).
- No particular effect differences by race.
- The efficacy across 6 different mutations shows some difference, but not a lot. The worrisome cases appear to be CAL.20C and D614G where the 95% confidence interval drops down to around 0%. (NB: the negative lower bound in the CAL.20C case is evidence that, like Pfizer, they’re using the Clopper-Pearson method for proportion uncertainty, but for some reason don’t know about the special case for near-zero counts. Just substitute 0% in your mind and move on.)
Safety
 The safety results show adverse events were mostly grade 1-2 (annoying to very annoying,
but not enough to hospitalize), and resolved within a day or two. Basically you might
feel like crap for a day or so, but it gets better fast. More good news.
The safety results show adverse events were mostly grade 1-2 (annoying to very annoying,
but not enough to hospitalize), and resolved within a day or two. Basically you might
feel like crap for a day or so, but it gets better fast. More good news.
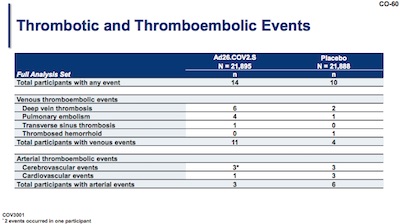
There’s some slight possibility of increased blood clotting problems (“thrombotic and thromboembolic events”). But with only 14 out of 21,895 in the treatment arm and 10 out of 21,888 in the control arm, it’s both rare and unlikely to prove statistically significant. I couldn’t resist doing the test of proportion myself; as you can see the result below has $p \sim 54\%$ (not at all statistically significant):
> prop.test(x = c(14, 10), n = c(21895, 21888))
2-sample test for equality of proportions with continuity correction
data: c(14, 10) out of c(21895, 21888)
X-squared = 0.37425, df = 1, p-value = 0.5407
alternative hypothesis: two.sided
95 percent confidence interval:
-0.0003016102 0.0006666983
sample estimates:
prop 1 prop 2
0.0006394154 0.0004568713
Still, the FDA scientists, in an abundance of caution, recommend monitoring for thrombotic events if an EUA is approved. Ok, cautious is good, I guess… so long as people get vaccinated.
Ok, it’s not quite as good as Pfizer and Moderna, but those were exceptional. This is very good, and has extremely strong logistical advantages over Pfizer and Moderna in rural areas or in the developing world. And remember: we must vaccinate all people in all countries, or the virus will continue to mutate until it evades our vaccines and comes back to bite us. In public health: “None of us is safe until everybody is safe.”
So let’s get everybody safely vaccinated.
A Twitter thread summary
Hilda Bastian has a Twitter thread in which she goes through the tables in the submission documents, reaching the same conclusions we did from the VRBPAC slide presentation. It’s worth reading, just for confirmation:
VRBPAC Verdict

Here’s the formal statement of the question before the VRBPAC, formulated by the FDA scientists who did the reanalysis of the J&J data. Basically, “does it help or hurt to approve this thing for emergency use in adults?”
Note that they did not make the mistake that Pfizer did, where they tried for approval in 16 years old and up. That triggers all sorts of headaches about pediatric trials, which were not done. Pfizer had some young folk in their trial, but the statistics were laughably uncertain due to small numbers. This was enough of a blunder that some of the VRBPAC members actually voted against the Pfizer EUA on this basis alone. So it’s good to know that Moderna didn’t repeat that mistake, and neither has Johnson & Johnson.
 Over at STAT News, Helen Branswell and
Matthew Herper (“the old reliables”) have been publishing notes in real time about the
VRBPAC meeting as they’ve watched it today. [11] It’s
pretty revealing about the topics that concern the VRBPAC members (one dose vs two,
severity of sickness, advese event analysis, why communicating with the public about
vaccines is difficult and about to get worse, anaphylaxis, …).
Over at STAT News, Helen Branswell and
Matthew Herper (“the old reliables”) have been publishing notes in real time about the
VRBPAC meeting as they’ve watched it today. [11] It’s
pretty revealing about the topics that concern the VRBPAC members (one dose vs two,
severity of sickness, advese event analysis, why communicating with the public about
vaccines is difficult and about to get worse, anaphylaxis, …).
Here’s their report on the verdict:
The vote
5:05 p.m.: It all comes down to this. The panel voted on only a single question:Based on the totality of scientific evidence available, do the benefits of the Janssen Covid-19 vaccine outweigh its risks for individuals 18 years of age and older?
The results:
22 Yes, 0 No
Unanimous! Now there will be a discussion on why the panelists think about their votes, which could be as important as the vote in determining how the vaccine is authorized.
The FDA scientists didn’t have to agree with Janssen; but they did. The VRBPAC didn’t have to agree with the FDA scientists; but they did. The FDA itself doesn’t have to agree with the VRBPAC; but they usually do.
For Pfizer and Moderna, the FDA formal EUA issued the same evening of the decision. (But some of that was Trump being an idiot making pointless blustery threats. At least that’s fixed now.) So… the way to bet is an EUA either tonight or at the worst early next week. If the formal issuance of the EUA happens tonight or over the weekend, then 4 million doses currently warehoused by J&J could start shipping by Monday.
Today was a good day.
Addendum at 8pm
 Later in the evening, the FDA itself issued a statement about the VRBPAC recommendation
for approval. [12] It wasn’t exactly a full EUA, but a
statement that they’d work rapidly toward that and that J&J can start in on executing
vaccine distribution plans:
Later in the evening, the FDA itself issued a statement about the VRBPAC recommendation
for approval. [12] It wasn’t exactly a full EUA, but a
statement that they’d work rapidly toward that and that J&J can start in on executing
vaccine distribution plans:
Following today’s positive advisory committee meeting outcome regarding the Janssen Biotech Inc. COVID-19 Vaccine, the U.S. Food and Drug Administration has informed the sponsor that it will rapidly work toward finalization and issuance of an emergency use authorization. The agency has also notified our federal partners involved in vaccine allocation and distribution so they can execute their plans for timely vaccine distribution.
So even though the official EUA might come next week, vaccines can start moving tonight.
Addendum 2021-Feb-27
 Well, that was pretty fast, as these things go: the FDA just issued the EUA for the
J&J vaccine. [13]
Well, that was pretty fast, as these things go: the FDA just issued the EUA for the
J&J vaccine. [13]
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the third vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The EUA allows the Janssen COVID-19 Vaccine to be distributed in the U.S for use in individuals 18 years of age and older.
If you dig in and actually get the EUA letter to Janssen from the FDA Chief Scientist Denise Hinton, the crucial paragraph is:
Based on these data, and review of manufacturing information regarding product quality and consistency, it is reasonable to believe that the Janssen COVID‑19 Vaccine may be effective. Additionally, it is reasonable to conclude, based on the totality of the scientific evidence available, that the known and potential benefits of the Janssen COVID‑19 Vaccine outweigh its known and potential risks, for the prevention of COVID-19 in individuals 18 years of age and older. Finally, on February 26, 2021, the Vaccines and Related Biological Products Advisory Committee voted in agreement with this conclusion.
Having concluded that the criteria for issuance of this authorization under Section 564(c) of the Act are met, I am authorizing the emergency use of the Janssen COVID‑19 Vaccine for the prevention of COVID-19, as described in the Scope of Authorization section of this letter (Section II) and subject to the terms of this authorization.
So now it’s official!
How do the vaccines compare?
There are now multiple vaccines, with different delivery mechanisms (mRNA and viral vector), somewhat different vaccine efficacy at preventing overall infection, different cold chain requirements, different supply availabilities, … complicated! How should we compare them and thereby make rational choices?
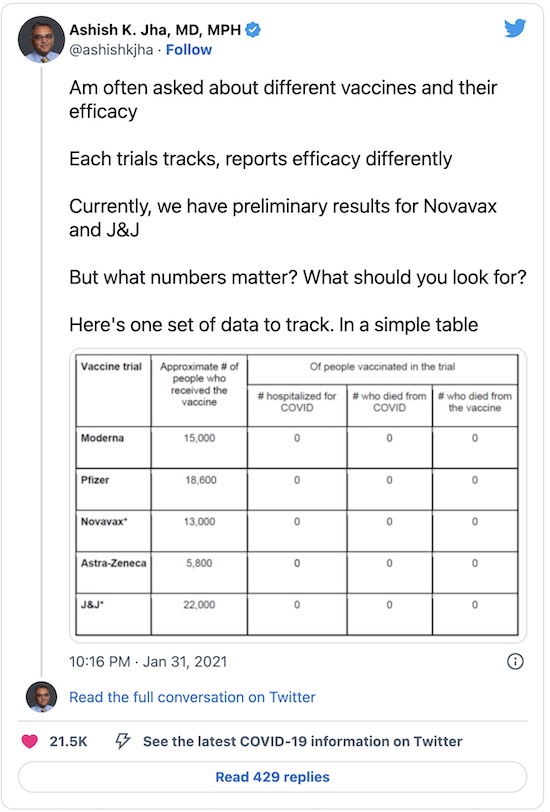
The best summary I’ve seen so far was from Ashish Jha, now dean of the School of Public Health at Brown, formerly of the Havard TH Chan School of Public Health (either of which would be an excellent capstone to a career; he has both, and is thus someone to whom we should listen carefully). He summarizes the results across 5 vaccines & vaccine candidates in a simple table:
Summary: There are 2 conclusions that he makes blunt-trauma-obvious here:
- Column 2: The vaccines have all been tested on large populations: $\geq O(10^4)$ trial subjects (except for AstraZeneca/Oxford, whose somewhat messed up trial and somewhat botched reporting we’ve detailed before; even they still look pretty good). That pretty much demonstrates they’re safe.
- Columns 3-5: Look at all those 0’s! Each vaccine might have somewhat variable efficacy in preventing all disease including even mild infections. But they are, as far as the clinical trial data can show, 100% effective at preventing COVID hospitalization, COVID death, or vaccine-related death. (With the last one, he’s just yanking our chain about how safe vaccines are… but he’s welcome to do it since that chain periodically needs yanking, hard.)
So… yeah, they’re all good.
Somebody asked me a while ago which vaccine they should get. First, because of supply constraints and the need to get everybody through the vaccine process, you probably won’t get a choice. Second, the data above shows the best vaccine is the first one you’re offered. Take the shot, thank the person who gives it to you, and smile.
What does it all mean?!
The J&J vaccine is good enough.
It may slightly inferior to Moderna and Pfizer or it may not be: Moderna and Pfizer were tested before some of the gnarly SARS-CoV-2 variants started appearing. Only a Phase IV trial (post-approval monitoring of people who get each vaccine) will tell, and that will take upwards of a year, maybe. So good enough will do here.
Also, J&J has remarkably superior logistics: cold chain requirements are much more lax, shipping is easier, a single dose is much less effort when you only have to see your patient once, and so on. (Though your humble Weekend Editor remains optimistic that the 2-dose trial of the J&J vaccine will reveal efficacy north of 90% and everybody will get a second dose anyway.) That alone makes the J&J vaccine a clear winner in rural parts of the US or in the developing world.
Addendum 2021-Mar-02
Shipping is in progress today; deliveries begin tomorrow:
Notes & References
1: M Herper & H Branswell, “FDA scientists endorse J&J’s Covid vaccine, as new data shed light on efficacy”, STAT News, 2021-Feb-24. ↩
2: D Lowe, “The J&J Vaccine at the FDA”, In the Pipeline blog at Science Translational Medicine, 2021-Feb-24.↩
3: D Lowe, “How You Make an Adenovirus Vaccine”, In the Pipeline blog at Science Translational Medicine, 2021-Feb-08.↩
4: D Lowe, “Myths of Vaccine Manufacturing”, In the Pipeline blog at Science Translational Medicine, 2021-Feb-02.↩
5: R Zhang & Y Hefter, “FDA Review of Efficacy and Safety of the Janssen COVID-19 Vaccine Emergency Use Authorization Request”, FDA/CBER Office of Vaccines Research and Review, 2021-Feb-26. ↩
6: M Allende, “Emergency Use Authorization: Overview and Considerations for COVID-19 Vaccines”, FDA/CBER Office of Vaccines Research and Review, 2021-Feb-25. ↩
7: Janssen Biotech, “COVID-19 Vaccine Ad26.COV2.S VAC31518 (JNJ-78436735): SPONSOR BRIEFING DOCUMENT”, Janssen Biotech/Johnson & Johnson, 2021-Feb-26. ↩
8: Janssen Biotech, “Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19”, Janssen Biotech/Johnson & Johnson, 2021-Feb-26. ↩
9: Janssen Biotech, “COVID-19 Vaccine Ad26.COV2.S VAC31518 (JNJ-78436735): SPONSOR BRIEFING DOCUMENT ADDENDUM”, Janssen Biotech/Johnson & Johnson, 2021-Feb-26. ↩
10: J van Hoof, H Schuitemaker, M Douoguih, & G Poland, “Emergency Use Authorization (EUA) Application for Ad26.COV2.S”, Janssen Biotech/Johnson & Johnson, 2021-Feb-26. ↩
11: H Branswell & M Herper, “Tracking an FDA advisory panel’s meeting on J&J’s Covid-19 vaccine”, STAT News, 2021-Feb-26. ↩
12: J Woodcock & P Marks, “FDA Statement on Vaccines and Related Biological Products Advisory Committee Meeting”, US Food & Drug Administration, 2021-Feb-26. ↩
13: US Food & Drug Administration, “FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine”, FDA Press Releases, 2021-Feb-27. ↩
Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.