Another beautiful vaccine: a look into the Moderna FDA EUA submission package
Tagged:COVID
/
MathInTheNews
/
PharmaAndBiotech
/
Politics
/
Statistics
Somebody asked me about the contents of the Moderna EUA application for their COVID-19 vaccine. Summary: It is also quite beautiful.
I woke up this morning, blearily lying abed listening to NPR. They said the Moderna EUA application at the FDA had dropped today, in preparation for the advisory committee (VRBPAC: Vaccines and Related Biological Products Advisory Committee) meeting on Thursday. So I thought, slowly and sleepily, “Yeah, probably ought to look into that sooner or later, maybe this afteroon.” After a bit of breakfast, I checked email and discovered that Gary Cornell had not only already found the FDA briefing docs, but had dug into them, had questions about the confidence intervals, and thought maybe I should have an opinion already, as well!
Adjusting for time zones, that was 6am for him when he emailed me, meaning he’d gotten up at around 5am his time to read the briefing docs while I was snoring. I’m a little bit intense, but I’m not 5am intense!
But… better late than never. Let’s have a look, now that it’s a civilized time of day.
What’s the sitch?
 Our first stop, as usual, is STAT News for a summary of the
situation. [1] As we’ve explained before, the US has the
quaint custom of throwing out the analysis & conclusions of drug applications, and
instead doing an ab initio analysis of their own on the data using the analysis methods
in the clinical trial protocol. Only then, analysis in hand, will they compare their
results with those of the submitter and proceed further if there’s agreement on what the
data say.
Our first stop, as usual, is STAT News for a summary of the
situation. [1] As we’ve explained before, the US has the
quaint custom of throwing out the analysis & conclusions of drug applications, and
instead doing an ab initio analysis of their own on the data using the analysis methods
in the clinical trial protocol. Only then, analysis in hand, will they compare their
results with those of the submitter and proceed further if there’s agreement on what the
data say.
That’s where we are today:
- The FDA agrees with Moderna that the trial is evidence that the vaccine is “highly effective” at preventing symptomatic COVID-19 14 days after the 2nd dose. (Asymptomatic COVID-19 is another matter, still under intense study. But see the Moderna supplement below, which has some intriguing early asymptomatic results.)
- The FDA also agrees that the side-effects will make you feel like crap for a day or so (though they didn’t print those exact words, I’m quite certain they were spoken), and that the degree might be a bit worse than Pfizer, but not enough to impede EUA approval.
- The FDA also finds evidence of partial immunity after 1 dose, but this is preliminary and in no way guidance for people to skip the second dose.
The Moderna FDA EUA application briefing documents
There are 3 documents this time, because Moderna filed a supplement:
- The Moderna submission (84 pages). [2]
- A supplement to the Moderna submission, because apparently you can’t amend the original document (7 pages). [3]
- The FDA reanalysis of the clinical trial data using the analysis methods in the protocol (54 pages). [4]
Fine, but what’s in those briefing documents?!
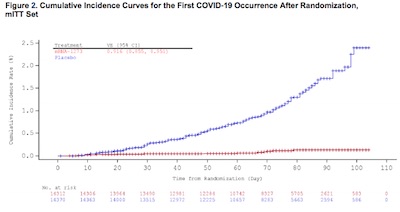 Remember in our look at the Pfizer/BioNTech EUA submission, there was
a very beautiful survival plot of infection rates in the treatment vs control arms?
Unsurprisingly, Moderna has one that is equally beautiful on page 28 of the FDA analysis,
Figure 2. The comments that come to mind are about the same as for Pfizer/BioNTech:
Remember in our look at the Pfizer/BioNTech EUA submission, there was
a very beautiful survival plot of infection rates in the treatment vs control arms?
Unsurprisingly, Moderna has one that is equally beautiful on page 28 of the FDA analysis,
Figure 2. The comments that come to mind are about the same as for Pfizer/BioNTech:
- The horizontal axis is time. The first injection occurred on day 1, and the second on day 29.
- The vertical axis is the frequency of COVID-19 cases seen, as a fraction of the number of subjects in each treatment arm.
- The red curve is the treatment arm (i.e., the vaccine), and the blue curve is the control arm. NB: These colors are reversed from Pfizer’s color choices, as there is no standard on these matters.
Note that the blue arm rises somewhat faster than linearly in time. That means there is an increasing probability/unit time, or rate, of being infected. This is the background case rate we would like to escape. (In Pfizer’s plot, this curve was more or less linear.)
The red arm, though: it shows more or less no protection up until about day 12, but then goes almost absolutely flat. It completely squashes the case rate to almost nothing. Yes, there are a couple cases; but remember there are about 15,000 people in each arm, so the probability/time, or rate, of getting infected is driven down to almost nothing beyond a few rare events.
This is very, very good! Especially hopeful is that the Pfizer/BioNTech and Moderna vaccines are quite similar technology, this represents an out-of-sample, independent confirmation of both vaccine’s efficacy. Things are looking up, I think.
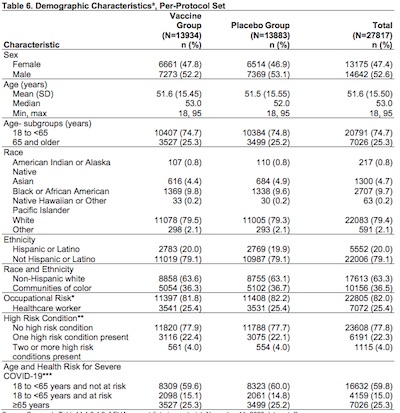 Table 6 on p. 20 gives us the subgroup breakdown of the trial population.
Table 6 on p. 20 gives us the subgroup breakdown of the trial population.
- Subjects: 13,934 in treatment group, and 13,883 in control group, so it’s well powered.
- It looks like the age range is 18 - 95 years of age, with about 25% of subjects over 65. So that’s good, they’ll get a good look into the response of older people.
- The racial makeup is 80% whites, about 10% Blacks, 5% Asian, and a miscellany of others. I wish it could have been a little less white, but that does include Hispanics, so maybe not so bad. It breaks down to 20% Latinx and 80% not; there were 63% non-Hispanic white.
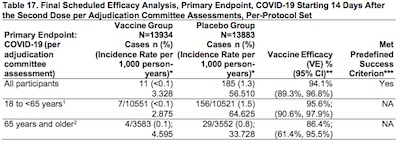 The real meat of the matter is in Section 5.2.5: Vaccine Efficacy, starting on page 22.
There are a number of interesting tables here, but we’ll concentrate on Table 17 (page
29): final efficacy analysis of the whole cohort, after both doses, with age breakdowns.
The efficacies and their 95% upper & lower confidence limits (very properly using a
confidence interval from a Cox proportional hazard method, in contrast to Pfizer where
they used the Clopper-Pearson method) are:
The real meat of the matter is in Section 5.2.5: Vaccine Efficacy, starting on page 22.
There are a number of interesting tables here, but we’ll concentrate on Table 17 (page
29): final efficacy analysis of the whole cohort, after both doses, with age breakdowns.
The efficacies and their 95% upper & lower confidence limits (very properly using a
confidence interval from a Cox proportional hazard method, in contrast to Pfizer where
they used the Clopper-Pearson method) are:
| Cohort | Efficacy | 95% LCL | 95% UCL | |||
|---|---|---|---|---|---|---|
| All | 94.1% | 89.3% | 96.8% | |||
| 18-64 yr | 95.6% | 90.6% | 97.9% | |||
| 65+ yr | 86.4% | 61.4% | 95.5% |
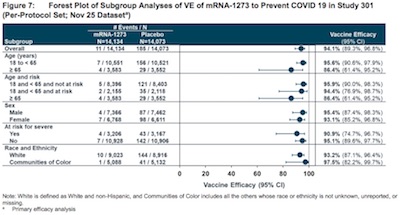 There’s a forest plot using the hazard ratios
and relating those to vaccine efficacy, on page 53 of the Moderna submission which
summarizes all of this graphically, with breakdowns by age group.
There’s a forest plot using the hazard ratios
and relating those to vaccine efficacy, on page 53 of the Moderna submission which
summarizes all of this graphically, with breakdowns by age group.
So it’s excellent overall, and still pretty good for those over age 65. The only worrisome spot is that we can’t really exclude the possibility that the efficacy is as low as 61.4% in elders. Still… that’s pretty good?
A note on confidence intervals
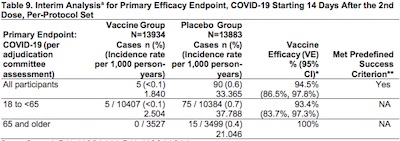 Gary had a few pointed questions about some of the other tables, in which there were no
confidence intervals for various subsets with age over 65. Tables 9 & 10 on pages 23
and 24 of the FDA report (interim analyses) are good examples. See in the 3rd row &
4th column where it just reports 100% efficacy with no confidence interval, unlike the
rows above it.
Gary had a few pointed questions about some of the other tables, in which there were no
confidence intervals for various subsets with age over 65. Tables 9 & 10 on pages 23
and 24 of the FDA report (interim analyses) are good examples. See in the 3rd row &
4th column where it just reports 100% efficacy with no confidence interval, unlike the
rows above it.
The clue to what’s going on here is to note that in the corresponding analysis by Moderna, the confidence interval is reported as (NE, 100%) where “NE” stands for something like “not evaluable”. This happens in every instance where there were no infections in the treatment arm (e.g., row 3, column 2 in the table shown here).
I suspect their confidence interval calculation method, based on the 95% CI of a Cox proportional hazard method, breaks down when the number of reported events is 0.
This is very like the situation in the Pfizer report, where they chose to use the Clopper-Pearson method (puzzlingly slightly inappropriate for a survival problem like this). There’s a special case for Clopper-Pearson when the reported events are 0% or 100% that gives an exact answer. If you use the interior Clopper-Pearson method, you get nonsense: either a negative lower confidence limit or an upper confidence limit above 100%. Hence the embarassing negative numbers in the Pfizer tables.
Here, Moderna specified in the trial protocol that they were going to use the more sophisticated Cox proportional hazards method (and properly so). But the penalty is when you observe 0 infections, you apparently can’t calculate a confidence interval. (Or so it seems. I haven’t checked out the math to be sure that this is the case.)
So… nothing really wrong there. And when you look at the full dataset as we did above, there were no zeroes anyway, so the problem did not arise.
The “problem”, if you really want a problem, is that the lower confidence limit for subjects age 65+ could be as low as 60%-ish. That’s still pretty good, though since your humble Weekend Editor is in that age group, it would be nice if it were higher.
So… what’s it all mean?
It means we have 2 independent randomized clinical trials on variations along the same mRNA theme. This is the gold standard as far as medical evidence goes, and it says both of Pfizer and Moderna appear to have excellent vaccines.
- Pfizer puts more onerous demands on the cold chain, and
- Moderna may have slightly more annoying side effects.
Other than that, I see little difference to convince me to prefer one over the other. I’d happily take either one today. There’s every reason to expect a favorable result when Moderna goes before the VRBPAC this Thurday. At that point, the vaccination capability of the US (and, for that matter, the world) will approximately double.
Be happy about this. It’s a good thing, in a year where good things have been lacking.
Notes & References
1: M Herper & D Garde, “FDA scientists endorse Moderna Covid-19 vaccine, as documents provide new hints on efficacy”, STAT News, 2020-Dec-15. ↩
2: Moderna Therapeutics, “mRNA-1273 Sponsor Briefing Document”, FDA.gov, downloaded 2020-Dec-15. ↩
3: Moderna Therapeutics, “mRNA-1273 Sponsor Briefing Document Addendum”, FDA.gov, downloaded 2020-Dec-15. Discusses duration of follow-up of subjects, adverse reaction definitions for lymphadenopathy (swollen lymph glands, usually in the armpit), some interim data on using Kaplan-Meier estimators for recurrence under intent-to-treat rules, and, most interestingly to me, some evidence supporting efficacy vs asymptomatic infection! ↩
4: FDA staff, “FDA Briefing Document: Moderna COVID-19 Vaccine”, FDA.gov, downloaded 2020-Dec-15. ↩
Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.