COVID-19: One Month Later
Tagged:COVID
/
PharmaAndBiotech
It’s been a month since we were exposed to COVID-19 here at Château Weekend, testing positive 2 days later. Surely it must all be over, right? Right? Ahem.
The sound of fat ladies not quite singing
The opera ain’t over until the fat lady sings. – Ralph Carpenter, then Texas Tech sports information director, quoted in the Dallas Morning News 1976-Mar-10. [1]
Fat ladies are not, as yet, singing. Or at least, they are singing very softly.
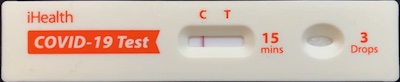 Yes, we both test negative. Yes, symptoms have largely abated. But we’re both weak as
kittens, and get utterly, deathly tired & achy & crabby by mid-afternoon. Not
just “tired”, but having actual difficulty cooking dinner because even standing up to eat feels
like a lot of work.
Yes, we both test negative. Yes, symptoms have largely abated. But we’re both weak as
kittens, and get utterly, deathly tired & achy & crabby by mid-afternoon. Not
just “tired”, but having actual difficulty cooking dinner because even standing up to eat feels
like a lot of work.
While we’re not totally incapacitated, having to work for a living would be difficult. Fortunately, your humble Weekend Editor is retired, and the Weekend Editrix works mostly from home. There are a number of blog posts that should have happened, except I can’t do anything other than sit and stare at a wall after 2pm.
But… things are getting better. Slowly.
Other things that are getting better
The world is still in the grips of climate change causing droughts severe enough to make parts of even the developed world uninhabitable. The incredibly stupid Russian invasion of Ukraine continues insensibly. The world slouches toward fascism. Trump is not yet indicted, let alone imprisoned.
So yeah, we got problems.
But at least a couple things are going well, or at least in the right direction:
-
Reuters reports [2] that the FDA has asked Pfizer to run a clinical trial to test the utility of a 2nd course of paxlovid in people who get rebound infections. As you’ll recall from our experience of rebound infection here at Chez Weekend, we strongly support this. (Yes, it’s COVID-19 rebound, not paxlovid rebound, since the rebound rates with and without paxlovid are statistically indistinguishable.) Still, when we asked for a second course of paxlovid, our doc fell back on the “CDC says there’s no evidence” line. In spite of all the mechanistic evidence and case report evidence. So it’ll be good to see a test of longer courses of paxlovid to shut down the virus and keep it shut down.
The trial is supposed to have its design finalized next month, which means if everything marches along smartly we can expect a readout by the end of the year and an FDA/CDC decision early in 2023. Yes, we all wish it were faster than that.
Ideally, I’d just like them to test a 10-day course of paxlovid for everybody, instead of 5 days + 5 more days if you get a rebound and your doc is up-to-date and feels like being cooperative. Just because expecting the healthcare system to respond to complexity is… expecting too much.
And frankly, we now know rebounds are pretty common, with our without paxlovid. And here at Chez Weekend, we know they’re no fun.
-
Pfizer and BioNTech have submitted to the FDA the EUA application for their bivalent booster vaccine. [3] (Yes, that’s a press release. And yes, we hate press releases here at Chez Weekend. But sometimes, it’s all ya got.) They are also doing rolling submissions with the EMA for European approval. They’re following the FDA guidance from this summer about which we blogged last June, and have begun “at-risk” manufacturing so they’ll be able to ship immediately upon grant of EUA. (I checked the FDA advisory committee calendar, and as of today see no VRBPAC meeting about EUA for Omicron-specific vaccines, either Pfizer or Moderna. Get on that, will ya, FDA folk? Thanks.)
It’s described as bivalent, albeit in a peculiar way:
The bivalent vaccine contains mRNA encoding the original SARS-CoV-2 spike protein, which is present in the original Pfizer-BioNTech COVID-19 Vaccine, together with mRNA encoding the spike protein of the Omicron BA.4/BA.5 variant.
This makes me wonder: if it contains spike mRNA from the classic, Omicron/BA.4, and Omicron/BA.5 variants, isn’t that trivalent? Or do the latter 2 variants share an identical spike protein? In any case, it looks to be generating strong antibodies to the original, Omicron/BA.1, Omicron/BA.4, and Omicron/BA.5 variants. Given the crosstalk of the original vaccination, it probably gets the variants inbetween, as well, though that does not seem to have been measured.
Moderna is expected to follow quickly. I’ve been impressed all throughout the pandemic that the fast, nimble biotech Moderna is always 2nd to apply for approval after the stodgy, slow big pharma Pfizer. I’d love to know why that is.
(But I’m grateful they’re both there. Given my recent deeply unpleasant COVID-19 experience, even with vaccination and paxlovid, they’re probably the reason I’m still here.)
Alas, this upcoming booster is probably the last one that will be free, unless Congress acts. And you know how hard it is to get Congress to do anything, with so many Republicans ready to obstruct everything. So after this, the drug companies will set prices and the insurance companies will decide how much we pay.
Happy nightmares on that front.
The Weekend Conclusion
So, at least a couple things are headed in the right direction.
Now if only we can blow up enough Russian military equipment, indict/try/convict Trump and his Republican enablers, keep the House for the Democrats & add 2 vertebrate Democrats to the Senate to neuter Senators Manchin & Sinema [4], then we’ll be in a position to make some real progress. For the first time in many years.
A tall order!
Notes & References
1: D Pincus, “Today in Sports History: March 10th”, SB Nation, 2010-Mar-09. ↩
2: L Leo, “FDA asks Pfizer to test second Paxlovid course in patients with COVID rebound”, Reuters, 2022-Aug-19. ↩
3: Pfizer & BioNTech Media Relations, “Pfizer and BioNTech Submit Application to U.S. FDA for Emergency Use Authorization of Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine”, Pfizer Press Releases, 2022-Aug-22. ↩
4: Am I the only one who longs to merge “Manchin” and “Sinema” into “machinima” and hope for a brilliantly satirical animation?
Nobody?
Ok, just me then. ↩
Gestae Commentaria
BA.4 and BA.5 have very similar spike proteins, sharing mutations at L452R and F486V. BTW, thanks for your blog and for posting your experiences.
First, welcome to the Weekend Commentariat!
Second, thanks for the info on the BA.4 and BA.5 spike proteins. This would explain why people always say “BA.4/5” (other than just sloppy terminology). Still, they’re not identical, so I wonder which one is in the vaccine or if they used both?
I appreciate your diligence in digging this up for me. Normally, I’d just say I was being lazy. But the post-COVID-19 fatigue and (essentially) brain fog have really slowed me down. So glad to hear someone’s checking the details for me.