The Weekend Editrix's Flu & Bivalent Booster Adventures (And Why She Went Through Them)
Tagged:COVID
/
JournalClub
/
PharmaAndBiotech
Today the Weekend Editrix got her annual flu vax and COVID-19 bivalent booster. Let’s look at why that’s a good idea for all of us.
[NB: This post is about events of 2022-Nov-21, but is posted somewhat delayed by several days. Thanksgiving holiday here in the US, you know!]
Where COVID-19 Antibody Technology May Be Going
The current mRNA vaccines are absolutely astounding: usually you’re looking at 5-10 years, and we got vaccines of > 90% efficacy (initially) within about 9 months! That’s just flabbergasting.
However, it’s not the last word. There are lots of developments! None of the existing antibody therapies work any more against Omicron/BA.4-5; let’s look through the literature to see what the future might bring in the way of antibodies.
A super-duper antibody
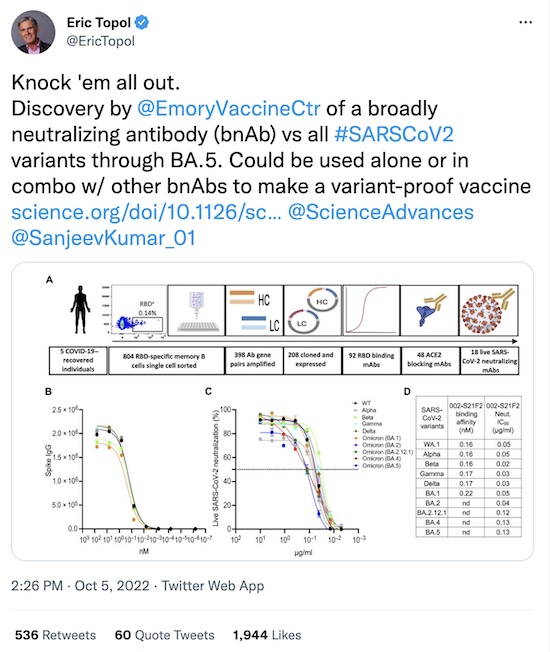
First, the indispensable Eric Topol alerts us to a broadly neutralizing antibody (bnAb) that works on all SARS-CoV2 variants through Omicron/BA.5:
 He’s pointing us to a Science Advances paper [1]. The
practical upshot is:
He’s pointing us to a Science Advances paper [1]. The
practical upshot is:
- If we can make this antibody (eventually, we can) then it can be used as a therapy. There are no remaining effective antibody therapies, so this is important!
- If we can create a vaccine which causes the body to produce this antibody (maybe?) then we can make a broadly effective vaccine, also important!
How was it found? Not as a result of any structural biology/protein trimer docking calculation, but empirically!
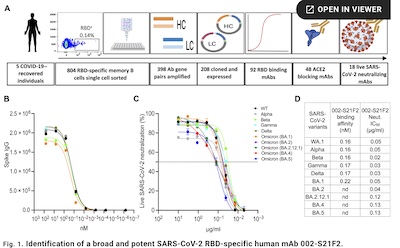 They extracted antibodies from 42 convalescent patients in India (all with the ancestral
Wuhan WA.1 strain in 2020), and just tested the antibodies. This one targets a
conformationally conserved (infrequently mutated) epitope on the outer face of the
receptor binding domain (RBD) where it grabs ACE2.
They extracted antibodies from 42 convalescent patients in India (all with the ancestral
Wuhan WA.1 strain in 2020), and just tested the antibodies. This one targets a
conformationally conserved (infrequently mutated) epitope on the outer face of the
receptor binding domain (RBD) where it grabs ACE2.
- Start with 42 patients, narrowed down to 5 with high SARS-CoV2 RBD binding titers.
- Select 398 antibodies, of which 208 were cloned & expressed.
- 92 had their RBD binding curves measured, getting 48 hits that block ACE2 binding.
- Of those, 18 had good neutralizing curves against live viruses.
The one so euphoniously yclept “002-S21F2” was the best of breed, as shown here in their Figure 1. There’s a lot more in the paper, in terms of assays and characterization of the immunologic properties of this antibody. But the bottom line is that they’ve found a very beautiful antibody that works very broadly against the then-extant variants.
It’s a hopeful sign that we might be able to manufacture this antibody and have an effective therapy again for those who can’t take paxlovid!
And… another antibody
Next, the equally indispensable Delthia Ricks alerts us to another broadly neutralizing antibody:

 She’s pointing us to a summary news article in News Medical [2],
which in turn points us to the primary source, an article in
Science Immunology. [3]
She’s pointing us to a summary news article in News Medical [2],
which in turn points us to the primary source, an article in
Science Immunology. [3]
Interestingly, this study works from a mouse model instead of sampling convalescent patients. This has pluses and minuses. They bred a mouse whose immune system slightly resembled a human’s (the primary B cell receptor (BCR) was generated through V(D)J recombination with a human VH1-2 heavy chain and human Vκ1-33 light chain). There’s a lot more going on there, but basically the mice respond in certain narrow immunological contexts like humans (sort of). So if you stimulate them with SARS-CoV2 spike protein, they might generate antibodies that would be of use to humans.
The big plus is that you can generate a lot of mice, infect/immunize them with the Wuhan variant’s spike protein (not the whole virus), and start harvesting a wide variety of antibodies to test. Their best was again “euphoniously yclept” SP1-77.
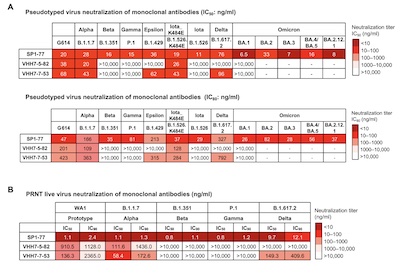 Here in their Figure 3 we can see how well it did. We’re looking at the neutralization of
two pseudotype viruses and the real virus, by 3 antibodies. The rows in the table are the
antibodies (SP1-77 is the first row), and the columns are viral variants. The number
tells us the IC50 in ng/ml units (lower is better, i.e., the antibody stops the virus at
lower concentrations. The columns in the table are viral variants. The color encodes the
concentration, with deeper/darker reds denoting more potent antibodies.
Here in their Figure 3 we can see how well it did. We’re looking at the neutralization of
two pseudotype viruses and the real virus, by 3 antibodies. The rows in the table are the
antibodies (SP1-77 is the first row), and the columns are viral variants. The number
tells us the IC50 in ng/ml units (lower is better, i.e., the antibody stops the virus at
lower concentrations. The columns in the table are viral variants. The color encodes the
concentration, with deeper/darker reds denoting more potent antibodies.
The thing to note is that SP1-77 had activity against all variants, with potency against the live virus ranging from 0.8 - 12.1 ng/ml. This is very, very good!
Better Vaccines
Now, antibodies are nice. Great, even, if we can manufacture them in quantity for infusions. Maybe greater if they guide us to better vaccines so we don’t need the infusions. So what’s on the horizon for vaccinations?
Bivalent spike/nucleocapsid vaccine
 Some months ago on this Crummy Little Blog That Nobody Reads,
we dissed the use of the viral nucleocapsid (N) protein.
It’s basically a terrible single target, since it’s
inside the viral capsule and thus not visible until after infecting a cell. As a
biomarker, it tends to indicate patients who had severe disease, not diverse immunity.
Some months ago on this Crummy Little Blog That Nobody Reads,
we dissed the use of the viral nucleocapsid (N) protein.
It’s basically a terrible single target, since it’s
inside the viral capsule and thus not visible until after infecting a cell. As a
biomarker, it tends to indicate patients who had severe disease, not diverse immunity.
In response, sharp-eyed commenter Mike pointed out an article in the LA Times [4] on using the nucleocapsid protein, not as a single target, but an additional target along with the spike protein (S). That’s interesting:
- A bivalent vaccine can use 2 proteins from the same virus.
- The N protein mutates less often (because it’s inside the viral envelope and subject to less evolutionary pressure), meaning immunity should be more stable with respect to the more frequent S mutations.
- Severe vaccine invasion might indeed require double mutations in both N and S, which is a very intriguing property.
- Animal studies seemed to point to T cell immunity more quickly, not just antibodies.
We followed this to the primary source, a paper in the big-time journal Science Translational Medicine. [5] Basically they tried a combined S and N gene mRNA vaccine, and compared with just the usual S mRNA. Some findings:
- Better protection against both Delta and Omicron as measured by viral presence in lungs & upper respiratory tract.
- Alterations in in vivo CD8+ T cells, suggesting increased T cell stimulation, validated by experiments with CD8+ T cell depleted hamsters.
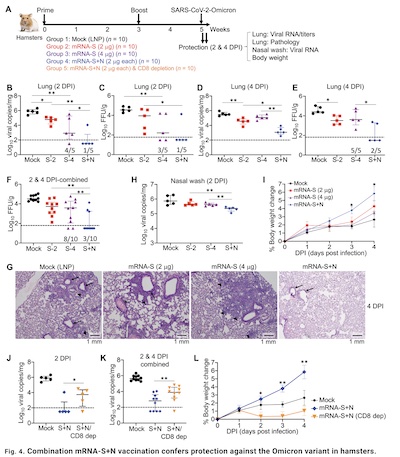 Their Figure 4 is the main deal here, showing the effect of control, S, and S+N
vaccination on hamsters exposed to Omicron:
Their Figure 4 is the main deal here, showing the effect of control, S, and S+N
vaccination on hamsters exposed to Omicron:
- Fig 4B: Note that log viral copies in the lung at 2 days post-infection is dramatically lower with S+N than with just S or nothing.
- Fig 4D: Same thing still holds at 4 days post infection.
- Fig 4H: The same thing holds for nasal washes at 2 days, indicating that the virus is still statistically significantly lower in the upper resipiratory tract.
- Fig 4J&K: Note that the effect partly goes away when given to CD8+ T cell depleted animals. This indicates that T cell immunity is being strongly stimulated and plays a positive role here, which is a very good thing to see.
Ok, so perhaps in the future we’ll see bivalent vaccines, not with 2 different strains of spike mRNA, but with spike and nucleocapside mRNA?
Combination COVID-19/flu vaccination


Currently, COVID-19 vaccines and flu vaccines are being offered at the same time. Not in the same shot, but at the same time and people are being encouraged to get them both at once. Not that there’s any particular reason to combine them, but you get better “patient compliance”: people will show up once, but probably not twice.
What if we could combine those 2 vaccines? Say, some mRNA for COVID-19 (maybe S+N, or even multiple variants) and flu? I mean, flu vaccines are already multivalent: the one I got this year had 4 different strains!
So it’s with some gratification that I came across a Reuters article and a Pfizer press release noting that Pfizer & BioNTech are starting a trial on exactly that. [6] [7] Moderna has been at it for a while, having announced their program almost a year ago, but they did mention it’s still ongoing in their most recent “R&D Days” review of clinical programs. [8]
So maybe next year, instead of getting multiple injections for COVID-19 and flu, we’ll just get one very complicated mRNA vaccine for both. Increased patient compliance means more people will be vaccinated. That’s a pleasant thought, no?
The present day: BQ.1 and BQ.1.1
 From the venerable Boston Globe comes a survey article [9]
warning us of new variants and all the havoc they are going to wreak in the near future,
mostly on those not fully vaccinated.
From the venerable Boston Globe comes a survey article [9]
warning us of new variants and all the havoc they are going to wreak in the near future,
mostly on those not fully vaccinated.
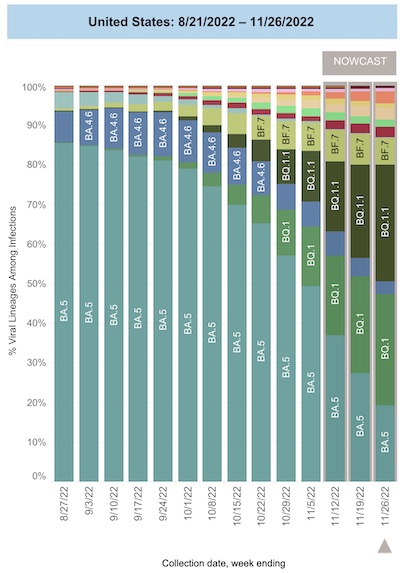 First, the CDC’s “nowcast” of SARS-CoV2 variants in the US is the canary in the coal mine:
First, the CDC’s “nowcast” of SARS-CoV2 variants in the US is the canary in the coal mine:
- We note that the fearsome Omicron/BA.5 (19.4%, CL: 17.1% - 21.9%) is on the wane, being replaced by BQ.1 (27.9%, CL: 25.5% - 30.5%) and BQ.1.1 (27.0%, CL: 27.0% - 31.9%). Given the fearsome aggressiveness with which BA.4/5 spread, this is impressive – and a bit scary.
- Think about it: the BQ variants together are 57.3% of the infections in the US, at least of the ones that show up in a hospital and are sequenced.
Alarmingly, all antibody therapies are now useless against COVID-19. This includes the latest bebtelovimab for treatment, and evusheld for prevention in those who cannot be vaccinated. Basically, we have vaccination, paxlovid, and non-pharmaceutical interventions (NPIs) like masking & social distancing.
Fortunately, hospitalization numbers are not rising. It appears BQ.1 and BQ.1.1 are replacing BA.4/5, but not causing new cases over and above what BA.4/5 would have caused. Apparently the background of vaccination and some post-infection immunity are helping us out there.
Knowing how the existing vaccines (including the bivalent booster tuned to BA.4/5) work
against BQ.1 and BQ.1.1 is essential. We know the antibody levels will be lower, but by
how much? Several studies paint a rather grim picture:
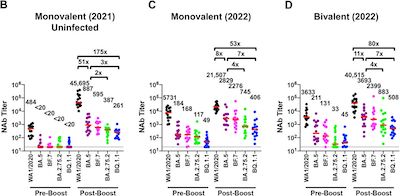
- A preprint from a group in virology & vaccines at the Beth Israel Deaconess Medical
Center [10] suggests a 7-fold reduction in antibody
levels vs BQ.1.1.
- Their Figure 1 parts B-D, reproduced here, show the neutralizing antibody titers vs
various SARS-CoV2 lineages for people with varying vaccination statuses.
- The most relevant part here at Chez Weekend is part D, where we’re looking at those with a bivalent booster.
- If you compare the levels against BA.5 (purple) vs BQ.1.1 (blue), you can see it’s labelled as a factor of 7x lower.
- Their Figure 1 parts B-D, reproduced here, show the neutralizing antibody titers vs
various SARS-CoV2 lineages for people with varying vaccination statuses.
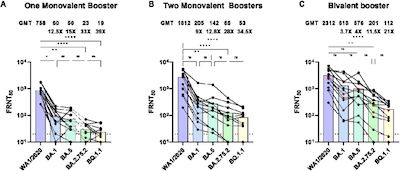
- A preprint from Emory broadly agrees. [11] Shown here is their Figure 1, which has their evidence. While the figure is hard to read, the paper reports levels for bivalently-boosted subjects in the FRNT assay of 576 for BA.5 vs 112 for BQ.1.1. So that’s a ratio of 5.14x reduction.
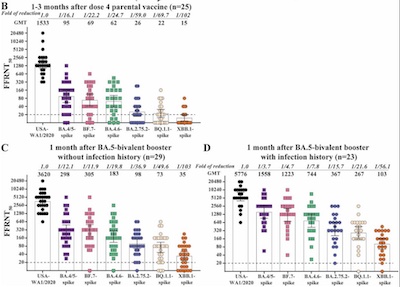
- Finally, a preprint from UTexas gets more or less comparable results. [12] Figure 1D tells the story for people with bivalent boosters. The figure’s a little easier to read here: 1558 for the BA.4/5 spike vs 267 for the BQ.1.1 spike. That’s a ratio of 5.83x reduction.
What does all this mean?
In the short term, you have something like 5-7x lower resistance to initial infection. In the longer term, you still have memory B cells that will spin up more antibodies and T cells that will fight off the infection and even kill infected cells. They’re a bit more slow than just having antibodies on hand, but they work. You’ll probably get infected, but fight it off quickly and only get a mild case. This is what vaccines are supposed to do!
Also, note that the reductions in antibody titers are for people who are fully vaccinated and boosted, with the new bivalent booster. If your vaccination is less than that, your antibody decrease is even worse, almost to the point where you have little protection at all. Strong incentive to get boosted with the bivalent booster!
More optimistic reports from the vaccine makers
Pfizer [13] and Moderna [14] of course have a sunnier view of things, as one might expect:
- Pfizer notes that the antibody levels vs BQ.1.1 go up 4.8 fold after the bivalent booster, but fail to point out that the level is still lower than for BA.5 or even the original virus.
- Moderna said the response was “robust” but didn’t support that with any data, other than noting it was 5x lower than for BA.4/5. This further cemented our prejucide here at Chez Weekend about the general uselessness of corporate press releases.
The venerable Globe wisely quoted Dan Barouch (the lab head behind the Miller paper [10], and director of the Center for Virology and Vaccine Research at BIDMC):
“The current vaccines are likely not going to provide substantial and sustained protection against infection, even with boosters,” Barouch said. “But these vaccines will likely still provide substantial protection against severe disease, and that is the most important goal of vaccines.” (Weekend emphasis added.)
The Weekend Conclusion
… and that’s why the Weekend Editrix today got her bivalent booster (Moderna) and an annual flu shot. We’re all vaxed up here at Chez Weekend, waiting for our immune systems to build immunity for the possible winter tridemic: another COVID-19 variant, influenza, and RSV.
Can’t do much about RSV, but we’ve done all we can about the other two.
You should too.
Notes & References
1: S Kumar, et al., “Structural insights for neutralization of Omicron variants BA.1, BA.2, BA.4, and BA.5 by a broadly neutralizing SARS-CoV-2 antibody”, Science Advances 8:40, 2022-Oct-05. DOI: 10.1126/sciadv.add2032. ↩
2: C Sidharthan, “All SARS-CoV-2 variants neutralized by a potent new antibody”, News Medical, 2022-Sep-09. ↩
3: Luo, et al., “An antibody from single human VH-rearranging mouse neutralizes all SARS-CoV-2 variants through BA.5 by inhibiting membrane fusion”, Sci Immunol 7:76, 2022-Aug-11. DOI: 10.1126/sciimmunol.add5446.↩
4: C Purtill, “Experimental COVID-19 vaccine could outsmart future coronavirus variants”, LA Times, 2022-Sep-14. ↩
5: RL Hajnik, et al., “Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models”, Sci Transl Med 14:662, 2022-Sep-14. DOI: 10.1126/scitranslmed.abq1945. ↩
6: Reuters Staff, “Pfizer, BioNTech start COVID-flu combination vaccine study”, Reuters, 2022-Nov-03. ↩
7: Pfizer Media Relations, “Pfizer and BioNTech Initiate Phase 1 Study of Single Dose mRNA-Based Combination Vaccine Candidate for Influenza and COVID-19”, Pfizer Press Releases, 2022-Nov-03. ↩
8: MB Woodin (Sr Dr R&D Comm), “MODERNA REVIEWS CLINICAL TRIAL PROGRAMS ACROSS PORTFOLIO AT 2022 R&D DAY”, Moderna Press Releases, 2022-Sep-08. ↩
9: R Cross, “A new coronavirus variant has taken over, sparking concerns of a winter surge”, Boston Globe, 2022-Nov-21. ↩
10: J Miller, “Substantial Neutralization Escape by the SARS-CoV-2 Omicron Variant BQ.1.1”, bioRχiv, 2022-Nov-02. DOI: 10.1101/2022.11.01.514722. ↩
11: ME Davis-Gardner, et al., “mRNA bivalent booster enhances neutralization against BA.2.75.2 and BQ.1.1”, bioRχiv preprints, 2022-Nov-01. ↩
12: C Kurhade, et al., “Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1, and XBB.1 by 4 doses of parental mRNA vaccine or a BA.5-bivalent booster”, bioRχiv, 2022-Nov-04. DOI: 10.1101/2022.10.31.514580. ↩
13: J Zou, et al., “Improved Neutralization of Omicron BA.4/5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent BA.4/5 Vaccine “, bioRχiv, 2022-Nov-17. ↩
14: C Ridley (VP Corp Comm & Media), “MODERNA’S BA.4/BA.5 TARGETING BIVALENT BOOSTER, MRNA-1273.222, MEETS PRIMARY ENDPOINT OF SUPERIORITY AGAINST OMICRON VARIANTS COMPARED TO BOOSTER DOSE OF MRNA-1273 IN PHASE 2/3 CLINICAL TRIAL”, Moderna Press Releases, 2022-Nov-15. ↩
Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.