Formal scientific publication of the Moderna COVID-19 vaccine Phase 3 trial
Tagged:COVID
/
MathInTheNews
/
PharmaAndBiotech
On 2020-Dec-30, the formal scientific publication of the Moderna’s COVID-19 Phase 3 trail in the prestigious New Englad Journal of Medicine dropped into public view. It’s good.
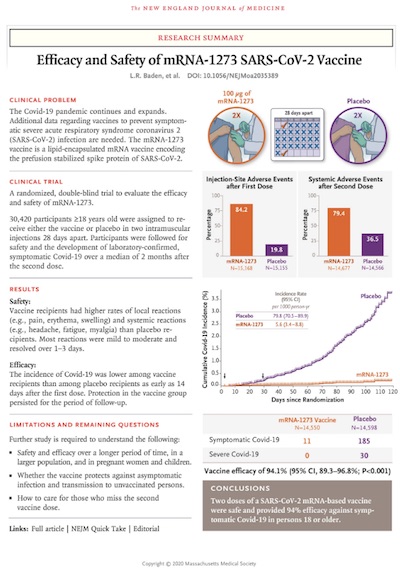 The Moderna COVID-19 vaccine is clinical trial
NCT04470427.
The Moderna COVID-19 vaccine is clinical trial
NCT04470427.
It has now been formally peer reviewed and is published in the prestigious NEJM. [1] The results confirm a vaccine efficacy (VE) result of 94.1% (95% CL: 89.3% – 96.8%). The supplement to the paper tells us that they calculated the VE very properly using a stratified Cox proportional hazards model, in which the probability ratio becomes a Hazard ratio, to account for trial censorship.
The efficacy in subgroups is exactly as we found in Figure 7 of the FDA submission: pretty good all-around, with some wider error bars in the very elderly.
This result is, as anticipated, very good indeed. There’s of course a lot more in the paper, including some lovely stuff about intent-to-treat vs as-treated doctrine, and all the usual stuff. But the top-line result of 94% efficacy is what we all want to see.
Notes & References
1: L Baden, et al., “Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine”, NEJM, 2020-Dec-30. DOI: 10.1056/NEJMoa2035389↩
Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.