Today I Got Shot Yet Again: A 6th COVID-19 Vaccination
Tagged:CatBlogging
/
COVID
/
PharmaAndBiotech
/
Politics
Today I got my 6th COVID-19 vaccination. Here’s why, in case you’re interested.
What happened this week in the US COVID-19 vaccine world?
Seems like we’ve been getting a lot of vaccinations here at Château Weekend! Since 2020, I’ve personally gotten 13 vaccinations: 6 COVID-19 + 3 flu + 2 shingles + 1 TDaP + 1 pneumonia. Today was the latest of those, a booster of the bivalent classic/Omicron COVID-19 vaccine.
Let’s look at what’s happened in US COVID-19 vaccination news to see why.
 There have, of course, been rumors for some months (as far back as late Feb/early Mar)
that a spring 2023 booster was a possibility. After all, they’ve already authorized it in
the UK and Canada. But all the reports were general media without sourcing to scientific
papers or regulatory bodies, and from reporters who had no particular qualifications that
I could easily discern. Maybe I need to read from better sources?
There have, of course, been rumors for some months (as far back as late Feb/early Mar)
that a spring 2023 booster was a possibility. After all, they’ve already authorized it in
the UK and Canada. But all the reports were general media without sourcing to scientific
papers or regulatory bodies, and from reporters who had no particular qualifications that
I could easily discern. Maybe I need to read from better sources?
However, this week it happened. Slightly strangely, I first heard of it in general media, which I then tracked back to the FDA news release. [1] A few high points:
- This did not include a meeting of the VRBPAC (Vaccine and Related Biological Products Advisory Committee) advisory committee. If that had happened, I would have been able to dig into a pile of presentations full of data. So they just went ahead and did it, without external advice. More on this below.
- This was done as an amendment to the Emergency Use Authorization for the bivalent vaccines of both Pfizer/BioNTech and Moderna. Unlike the monovalent vaccines, these are still under EUA, so it had to be this way.
- They completely eliminated the requirement to have the monovalent vaccine before the bivalent one, since the monovalent one is less useful vs Omicron anyway. This is sensible, though the slow speed at which it was done is not very sensible. So, overdue but good. But anybody still unvaccinated (why?!) will now start with the bivalent vaccines.
- There are still some complex rules around pediatric use, given that the Pfizer and Moderna pediatric trials were differently designed.
- The important bit: Anybody of 65 years old or immunocompromised who got a bivalent booster last fall is eligible for a spring booster now. Most people will get an annual booster in the fall, with a spring booster available for elders and immunocompromised people.
Thus, they’ve both authorized a spring booster for elders and immunocompromised, deprecated the now less useful monovalent vaccines, and simplified the rules for who gets what and when, based on risk exposure.
It looks like the J&J vaccine is pretty much dead in the US. The Novavax protein-based vaccine continues to struggle to show much advantage.


 This was then widely reported by various general news sources, most of which seemed to be
pretty accurate, if simplified. [2] [3]
[4] [5] [6]
[7] I often lean toward NPR, NYT, WaPo for general news.
But if you want short, then start with the Reuters report. Helen Branswell at STAT News
has done her usual fabulous job as well, giving a more detailed and deeply informed view.
This was then widely reported by various general news sources, most of which seemed to be
pretty accurate, if simplified. [2] [3]
[4] [5] [6]
[7] I often lean toward NPR, NYT, WaPo for general news.
But if you want short, then start with the Reuters report. Helen Branswell at STAT News
has done her usual fabulous job as well, giving a more detailed and deeply informed view.
But, of course, there’s nothing like the primary source, which in this case is the FDA release above.
Why they might have done that
Ok, but… I have questions:
- Why was there no VRBPAC meeting with presentations to the FDA?
- Why is a booster relevant now, in terms of risks of hospitalization and death?
- What are the alternatives, now that no antibody infusions work anymore (even Evusheld)?
So lets’s look at each of those in turn.
Why no VRBPAC?
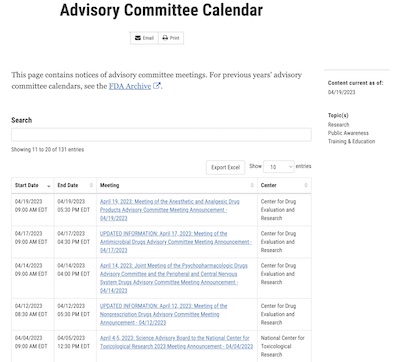 How do we know there was no VRBPAC meeting to advise the FDA? Because we looked at their
schedule [8], part of which is snapshotted here. Since the
FDA announcement above was 2023-Apr-18, we’d normally expect the VRBPAC meeting to have
been shortly prior to that. As you can see (click to embiggen), there was no such meeting
and we didn’t just miss it.
How do we know there was no VRBPAC meeting to advise the FDA? Because we looked at their
schedule [8], part of which is snapshotted here. Since the
FDA announcement above was 2023-Apr-18, we’d normally expect the VRBPAC meeting to have
been shortly prior to that. As you can see (click to embiggen), there was no such meeting
and we didn’t just miss it.
So why did the usually cautious FDA not do a VRBPAC? It’s a bit of a guess, but I can see at least 2 reasons:
- A spring bivalent booster has already been approved abroad, e.g., in the UK and Canada. [9] That means, among other things, the FDA has their approval data available to inspect, and needn’t duplicate the effort. (I haven’t personally checked, since I’ve no idea how to navigate the health regulatory agencies of those countries.)
- Also, there was a previous VRBPAC on 2023-Jan-10, about which we blogged at the time [10], on exactly this issue: composition of vaccines going forward and criteria for issuing a new booster. Provided this is reasonably in line with those plans, another VRBPAC may have been judged unnecessary.
What’s the risk-based need for a booster now?

 Is there a risk-based story here, i.e., an observable waning of protection and associated
risk roughly 6 months after the last bivalent vaccination?
Is there a risk-based story here, i.e., an observable waning of protection and associated
risk roughly 6 months after the last bivalent vaccination?
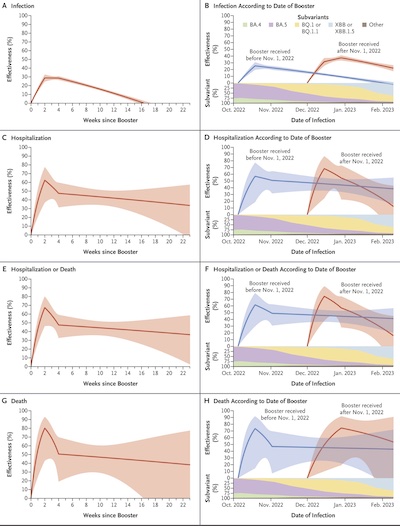
There are quite a few studies here, so I’ll just concentrate on one of them which studies the durability of various vaccine efficacies both over time and across various viral variants. It’s a very brief couple-page letter to the editor of the New England Journal of Medicine. [11]
Consider their Figure 1, which summarizes things:
- The left column looks at persistence of 4 kinds of efficacy (infection, hospitalization, hospitalization or death, and death) vs time.
- The right column does something similar, but doesn’t put all the initial vaccination dates at 0. Instead, by basing each curve on the calendar date, we get to compare efficacy with the various viral variants that were common at that time.
- Looking at the left column, we see that protection against infection has pretty much disappeared by 16 weeks (4 months). Sterilizing levels of antibodies are too low for that.
- However, we also see that protection against hospitalization and death, while reduced, persist quite a bit more. You might get infected, but you’re less likely to die. This is because once an infection happens, the body can spin up antibody production to fight it off.
- Looking at the right hand column, we compare the red and blue curves to see that the general levels of efficacy persist across viral variants. This is good, since we can discount the hypothesis that the experiment would not reproduce if tried at another time, with different variants.
What’s the story on antibody infusions?
 Previously, we had antibody infusions for treatment like bebtelovimab and
long-persistence antibodies like Evusheld for prevention. Those are both great, and
probably saved the life of a family member.
Previously, we had antibody infusions for treatment like bebtelovimab and
long-persistence antibodies like Evusheld for prevention. Those are both great, and
probably saved the life of a family member.
However, none of those work any more, in the era of Omicron.
In a bit of (occasional) news, AstraZeneca now has a new antibody [12] which appears to be effective. AZD3152 is not approved yet, but probably by the end of this year.
Ok, good news… but… it’s still the case that there are no treatment alternatives for people who get infected, at least for a while. With luck, that may change by the end of the year. In the meantime, we need prevention… i.e., a booster.
How about the CDC?
As apparently everyone knows by now, the FDA and CDC have different roles:
- The FDA decides what’s scientifically true, i.e., whether a proposed treatment is both safe and effective.
- The CDC looks at that, and if the FDA review is favorable, sets recommended standards for patient treatment. It’s more pragmatic than scientific.
So… what did the CDC have to say about this FDA finding for boosters? The CDC’s ACIP (Advisory Committee on Immunization Practice) met the very next day, which is gratifyingly fast.
 But their agenda was curious! [13] Note on the agenda,
reproduced here, that there was no opportunity for a vote! Why would you convene an
external advisory committee and not ask them for a recommendation?
But their agenda was curious! [13] Note on the agenda,
reproduced here, that there was no opportunity for a vote! Why would you convene an
external advisory committee and not ask them for a recommendation?
Perhaps… you just want to look for red flags. These vaccines have been reviewed about as much as any medication has ever been reviewed. Absent any red flags, you’ll approve the booster. It would be nice if we could accept an ACIP vote without prejudice, but if we really have a default toward acceptance, this might be a sort-of-ok way to do this.
Now, given that the FDA has already approved this without a VRBPAC, we’re not gonna go through the whole CDC ACIP meeting.
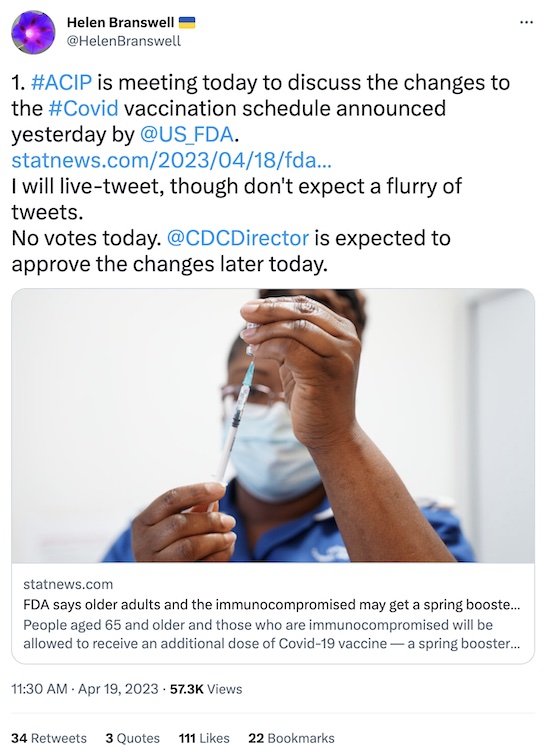
 Fortunately, the redoubtable Helen Branswell of STAT News has live-tweeted the entire
CDC ACIP meeting. I’m not going through all that, but she’s got what is apparently a
pretty good summary.
Fortunately, the redoubtable Helen Branswell of STAT News has live-tweeted the entire
CDC ACIP meeting. I’m not going through all that, but she’s got what is apparently a
pretty good summary.
At the end of the day, the CDC agreed with the FDA: older adults and the immunocompromised should [14] get a bivalent booster.
What to do about it
So we’ve seen that:
- The national health authorities of the Canada, the UK, and the US have all authorized another bivalent Omicron booster for the elderly and the immunocompromised.
- There is ample evidence of a 4-6 month waning against Omicron, depending on what sort of protection you measure.
- We’re over 65 here at Château Weekend (or at least soon will be), about 6 months has elapsed since our last bivalent vax, and our personal cost of having COVID-19 last August was high cognitive impairment that persists until today.
That’s general availability, evidence of broad need, and evidence of personal need.
The correct course of action is, of course, quite obvious: get the booster. Now the Weekend Editrix is (a) in Japan, and (b) a hair too young. The Weekend Publisher couldn’t be convinced to focus, and there are no feline vaccines yet.
 So, just me then. It was relatively straightforward: I used the government vaccine finder
web site, which pointed me at several CVS’s within a couple miles of Château
Weekend. So clicking through, it unfortunately took me to the national web site for CVS
and I had to re-enter a bunch of stuff. But it pretty quickly recognized me and gave me a
same-day 5pm appointment.
So, just me then. It was relatively straightforward: I used the government vaccine finder
web site, which pointed me at several CVS’s within a couple miles of Château
Weekend. So clicking through, it unfortunately took me to the national web site for CVS
and I had to re-enter a bunch of stuff. But it pretty quickly recognized me and gave me a
same-day 5pm appointment.
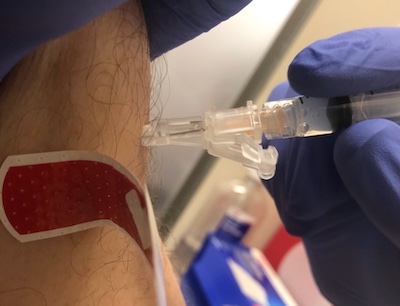
I got there about an hour early, and asked if they had a spot, since it wasn’t especially busy. They said “Sure, have a seat”, and about 10 minutes later a very friendly pharmacist gave me the Moderna bivalent booster. (I have a slight favorable attitude toward Moderna, since it’s dosed a bit higher than Pfizer. But that’s over-optimization on my part; just use whichever one’s available to you.)
And here’s the photographic evidence of my hairy dorsal manipulator tentacle getting a 6th COVID-19 vaccination since 2021.
The Weekend Conclusion
 As you can see here, the Weekend Publisher is nonplussed with all this, preferring to
remain on guard duty against the horde of wild turkeys so rudely stalking about his back
yard.
As you can see here, the Weekend Publisher is nonplussed with all this, preferring to
remain on guard duty against the horde of wild turkeys so rudely stalking about his back
yard.
While I admire his nonchalance, the rational way to attain that nonchalance is to take all the sensible precautions that science and common sense dictate. For us, that meant getting the boost when available.
Maybe you should think about doing likewise?
Addendum 2023-Apr-21: Side effects
Really not so bad. I was tired last night, and my arm was a little sore. Today I got up really tired, and a little achy. No fever, though.
Probably the easiest of the 13 vaccinations I’ve had since 2020. (6 COVID-19 + 3 influenza + 2 shingles + 1 TDaP + 1 pneumonia, in case you want the complete score.)
Notes & References
1: FDA Staff, “Coronavirus (COVID-19) Update: FDA Authorizes Changes to Simplify Use of Bivalent mRNA COVID-19 Vaccines”, FDA News Releases, 2023-Apr-18. ↩
2: M Erman & L Leo, “US FDA authorizes second Omicron-updated COVID booster for older adults “, Reuters, 2023-Apr-18. ↩
3: H Branswell, “FDA says older adults and the immunocompromised may get a spring booster dose of Covid vaccine”, STAT News, 2023-Apr-18. ↩
4: B Goodman, “FDA clears the way for additional bivalent boosters for certain vulnerable individuals”, CNN, 2023-Apr-18. ↩
5: L McGinley & L Sun, “FDA backs second omicron booster for high-risk groups”, Washington Post, 2023-Apr-18. ↩
6: C Jewett, “F.D.A. Authorizes Another Covid Booster Shot for People Over 65”, New York Times, 2023-Apr-19. ↩
7: R Stein, “FDA says some adults can get a second boost of the bivalent COVID-19 shot”, National Public Radio, 2023-Apr-18. ↩
8: FDA Staff, “Advisory Committee Calendar”, snapshotted 2023-04-20. Shows all meetings that occurred so far in 2023-Apr. NB the absence of any VRBPAC meeting. ↩
9: H Branswell, “FDA offers radio silence on question of spring Covid boosters, as other countries push ahead”, STAT News, 2023-Mar-16. ↩
10: Weekend Editor, “FDA VRBPAC: COVID-19 Vaccine Composition Going Forward”, Some Weekend Reading blog, 2023-Jan-27. ↩
11: DY Lin & SK Sunny, “Durability of Bivalent Boosters against Omicron Subvariants”, New England Journal of Medicine, 2023-Apr-12. ↩
12: M Fick, “AstraZeneca confident new COVID antibody protects against known variants”, Reuters, 2023-Apr-18. ↩
13: CDC Staff, “Final - April 18, 2023 MEETING OF THE ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP), Centers for Disease Control and Prevention Atlanta, Georgia 30329 April 19, 2023”, CDC Meeting Announcements, 2023-Apr-18. ↩
14: CDC Media Relations, “CDC simplifies COVID-19 vaccine recommendations, allows older adults and immunocompromised adults to get second dose of the updated vaccine”, CDC Newsroom Releases, 2023-Apr-19. ↩
Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.