What's in the Phase 1/2 readout of the Moderna/Lonza COVID vaccine?
Tagged:COVID
/
PharmaAndBiotech
/
SomebodyAskedMe
Ok, so I finally tracked down the official Phase 1/2 readout of the Moderna/Lonza COVID vaccine (whose Phase 3 delay we commented on about 10 days ago). Here are some quick thoughts, but the top line seems to be very good.
 Now, I know… I always grump about the media and their general mathematical and scientific
illiteracy. Still, like everybody else, I do read. In this case, I read actual,
old-fashioned newspapers in hardcopy. (That’s because I’m an actual, old-fashioned guy
still in corporeal hardcopy.) In this case, it was the venerable Boston Globe:
Now, I know… I always grump about the media and their general mathematical and scientific
illiteracy. Still, like everybody else, I do read. In this case, I read actual,
old-fashioned newspapers in hardcopy. (That’s because I’m an actual, old-fashioned guy
still in corporeal hardcopy.) In this case, it was the venerable Boston Globe:
J Saltzman, “Moderna’s COVID-19 vaccine prompted an immune response in all 45 trial subjects”, Boston Globe, 2020-Jul-14.
Ready for some good news?
The COVID-19 vaccine proposed Moderna Phase 1/2 trial has just read out. 45 patients in, 45 patients out with acceptable side effects and pronounced immune reactions. Before we dig out the primary documentation, this report in the venerable Boston Globe looks good.
Related: the Phase 3 trial (30,000 patients) starts July 21, i.e., next week. That’s on track to read out late in the year. If the result is positive, Moderna has partnered with Lonza (Swiss pharma firm) which has the vaccine manufacturing capabilities to source ~ 1bln doses/year.
Now, here’s the cautionary note about media accounts: never stop there! Always dig out the primary documents, and get hold of the real data. In this case, here’s the actually useful stuff on the readout of the Moderna Phase 1/2 vaccine trial, not just random newspaper articles:
- Semi-popular article in StatNews: M Herper, D Garde, “First data for Moderna Covid-19 vaccine show it spurs an immune response”, StatNews 2020-Jul-14.
- The actual scientific paper: L Jackson, et al., “An mRNA Vaccine against SARS-CoV-2 — Preliminary Report”, NEJM 2020-Jul-14.
- The registry of the Phase 3 trial at ClinicalTrials.gov (NCT04470427): A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19.
Looks about like the Pfizer/BioNTech trial we saw last week (unsurprisingly, as both are early safety trials):
-
Pool of normal, healthy, young volunteers – not the high-risk population, but entirely appropriate for an early safety study.
-
Everybody got some level of side-effects, but no SAEs. (“Severe adverse event”, where “severe” means “requires hospitalization”. But the observed AE’s were described as “unpleasant”, which means you probably really wouldn’t like it.)
-
Everybody showed antibodies. The level of antibodies was properly dose-dependent (increased with each of the 3 tested doses), and also increased at the second booster dose. This makes it more likely the antibodies are causally related to the vaccine.
-
The antibodies were serum-neutralizing, i.e., shown to act against SARS-COV-2 virus in vitro. Also, the antibody levels were higher than those observed in patients who have recovered from COVID-19, i.e., they should be at or above meaningful levels.
-
They tested 3 dose levels, and it’s the middle one that’s going forward in the Phase 3 trial. That’s promising, since it says they probably understood how to pick about the right dose already, and bracketed it in the Phase 1/2 trial. So there’s a good shot at having the right dose in the Phase 3.
-
This is the most advanced of the 23 vaccines currently in human trials (and hundreds in pre-clinical development).
-
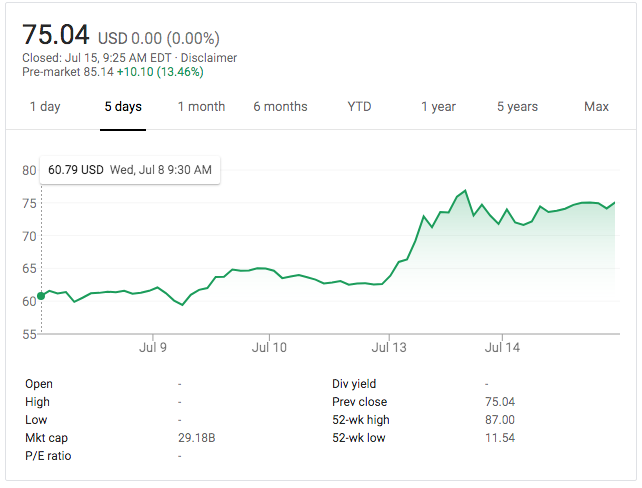
And here’s what happened to Moderna’s stock price yesterday (i.e., probably too late to get in today, and please don’t anybody take anything I say as investment advice, ever; just buy some index funds instead):
-
What’s not reported is the duration of the antibodies, and thus presmuably the disease resistance (though that has to be proven as well!). Here, they only looked out a couple weeks, as is appropriate for an early-stage safety trial. The Phase 3’s will go out to about 6 months of follow up before their data lock and possible submission. At that point, you’re in the cruel trade-off between knowing duration of response (good) vs delaying availability of the vaccine (bad). There are nice mathematical models using utility theory to tell you the QALY trade off, but in the end you have to decide to move or not — the models will just tell you not to be stupid, but not what the optimum is.
-
Also unknown is whether the abs work in vivo — as of now we only know that they bind the virus in vitro. The rate of infection in the Phase 3 will help us estimate that.

Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.