FDA VRBPAC: COVID-19 Vaccine Composition for Fall 2024
Tagged:COVID
/
JournalClub
/
PharmaAndBiotech
/
Politics
Today the US FDA’s Vaccine and Related Biological Products Advisory Committee (VRBPAC) met to discuss the composition of COVID-19 booster vaccines for fall 2024. Let’s check out the science content.
VRBPAC Meeting Announcement
People act as if COVID-19 is “over”. The virus begs to differ. We’re looking at summer and winter waves into the indefinite future. People will die of COVID-19, or – like your humble Weekend Editor – will have Long COVID brain fog and other problems.
Fortunately, our regulatory bodies are not ignoring this and are on the case for updated booster vaccines. Getting the public, even the non-knucklehead public who are not conspiracy theorists, to take the vaccines… well, that’s another matter. We can solve the vaccine design problem; thus far we have not solved the public vaccine uptake problem.
Today we look at the latest meeting of the FDA VRBPAC, to consider the strain composition of the vaccine for this fall.

We start, of course, with periodic monitoring of the FDA’s advisory committee calendar. [1]
There we see, postponed from 2024-May-16 to allow for more data collection, the VRBPAC meeting today on vaccine strain composition. [2]
At the bottom of that page are links to all the presentation materials, as well as a livestream on YouTube [3], for those of us who want to watch and hear all the discussion.
The Usual Administrivia
The usual boilerplate was boiled and plated thusly:
- There were 24 members, including temporary members, of which it appears 17 were in attendance. [4] (Though, as you’ll see below, there were 16 votes counted.)
-
Interestingly, there were no conflicts of interest reported for waivers! [5]
That’s a bit unusual, since almost always somebody has some stock, or a consulting agreement, or some research funding from industry. Not enough to be a red flag, but an interesting fact behind which there’s probably a story.


 The meeting agenda [6] contains the usual things:
The meeting agenda [6] contains the usual things:
- Start with introductions and administrivia.
- Most of the day will be scientific presentations.
- Then there’s an hour of public commentary (for which, by tradition, everybody pretty much goes away because it’s usually too much to bear for mere mortals).
- Finally the discussion and voting will be at about 2:30pm. It’s all about whether the current monovalent XBB.1.5 vaccination should be updated to the current strains (JN.1 lineage: JN.1, KP.2, KP.3, LA.2, …).
You’d think this should be pretty much a slam-dunk “yes” vote, but let’s see what actually happens: they’re allocating 2 hours for this discussion and voting.
The FDA Briefing Document
 There’s always a formal briefing document [7] filed by the
FDA itself for a meeting like this. It’s… dense, as is the custom of the tribe.
There’s always a formal briefing document [7] filed by the
FDA itself for a meeting like this. It’s… dense, as is the custom of the tribe.
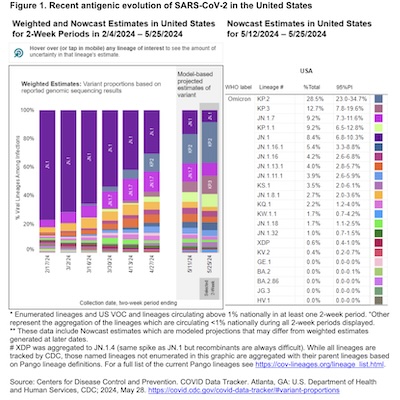
However, Figure 1 is outstanding. It tells us the current set of strains active in the US, and how they have changed over time.
- It shows each variant of concern (VOC) with circulating above 1% over 2-week periods from February through May. (It’s slightly unclear just what population is being measured: hospitalized patients (if so, where?), wastewater (from where?), etc. Probably digging into the CDC web site shown at the bottom of the figure would answer those questions.)
- In February, the JN.1 variant had completely displaced the XBB.1.5 variant that was the big noise last fall.
- However, by mid-May, JN.1 was itself crowded out by other variants. We’ll see later that these are in the same variant family as JN.1. The virus is honing the infectiousness of JN.1 at quite a rapid rate, to have this fast turnover.
This is graphic evidence that SARS-CoV2 is not done with humanity. It doesn’t help that we’ve given up any protective measures, and decided upon helplessness and ignorance as a strategy. This will not end well.
However, at least the the problem we can solve, vaccine composition, the course is clear. Target the JN.1 lineage, whether via monovalent or multivalent formulation.
 There was a (very) short presentation by Jerry Weir of the FDA [8],
documenting the FDA vaccine process as a flowchart, and asking the discussion question and
voting questions above. Seems pretty clear: the JN.1 lineage, broadly speaking, is the
current villain, and does everybody agree we should do something about that?
There was a (very) short presentation by Jerry Weir of the FDA [8],
documenting the FDA vaccine process as a flowchart, and asking the discussion question and
voting questions above. Seems pretty clear: the JN.1 lineage, broadly speaking, is the
current villain, and does everybody agree we should do something about that?
My prediction: everybody will agree on JN.1 family, but there will be argument over monovalent vs multivalent formulations, and which age groups get it when.
Science Presentations
Ruth Link-Gelles, CDC: Effectiveness of Current Formulation
 First up was Ruth Link-Gelles of the CDC and a familiar face at FDA VRBPAC and CDC ACIP
meetings on COVID-19 vaccines. She’s reviewing the efficacy of last year’s XBB.1.5
vaccine versus current strains. [9] This is an achingly
significant topic!
First up was Ruth Link-Gelles of the CDC and a familiar face at FDA VRBPAC and CDC ACIP
meetings on COVID-19 vaccines. She’s reviewing the efficacy of last year’s XBB.1.5
vaccine versus current strains. [9] This is an achingly
significant topic!
She’s got a lot to say, but the high points for me were on 5 of her slides:
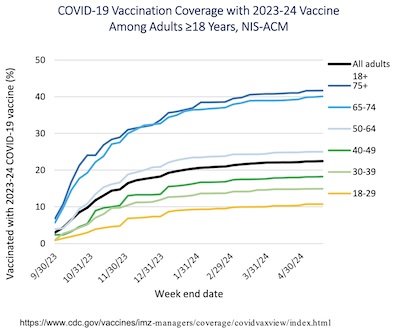
- First, booster uptake vs time and stratified by age was pretty miserable!
- Elders were most eager to get boosted, but among them only about 40% managed to do it.
- After that, the plateau after about 5 months of availability stratifies by age, with younger cohorts doing demonstrably worse. In fact, only about 10% of people in their 20’s managed to get boosted: they’re walking Petri dishes, passing the virus around, giving it human lab space to try out mutations.
- This is one reason why COVID-19 won’t go away. Really, to me the question is: are
we making it that difficult to get vaccinated, or do we keep it secret so nobody
knows they need it, or are we just stupid? (At least we can do something about the
first 2 possibilities. For the 3rd possibility, we can only resort to Schiller:
“Mit der Dummheit kämpfen Götter selbst vergebens”.
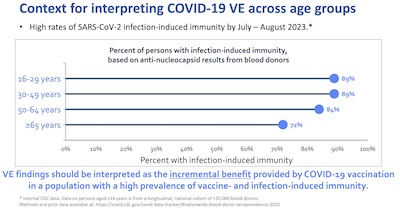
- Next, she goes on to point out that vaccine efficacy in this context means incremental immunity
over and above the already-existing immunity from previous vaccination or
infection. This makes a bit of a difference, especially among the young: they’ve been
reckless about vaccination & prevention, so they’ve been infected a lot, but that
means they have some more immunity. Boosters are especially important the older you
are.
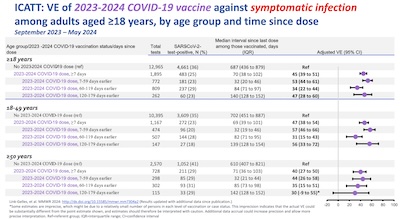
- Then she looks at the ICATT data, to see the protection vs symptomatic infection, by age
cohort and time since boost. We see that it’s about 50% (incrementally above
pre-existing immunity), though it declines faster in elders. After 6 months in those
over 50, the error bar overlaps 0, which is why we really want to see twice-yearly
boosters for those over 50.
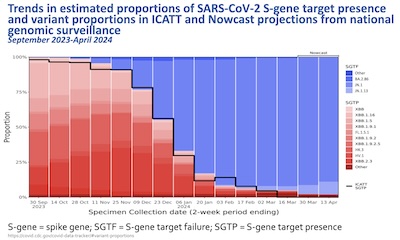
- But of course the virus is a genetic moving target! The sequencing methods using
primers from the older variants fail to detect part of the spike gene in the newer
variants. The plot here shows the rate of S-gene target failure, over time. This
exactly correlates with and explains the rise of the Omicron-descended variants JN.1,
BA.2.86, and others. They are different.
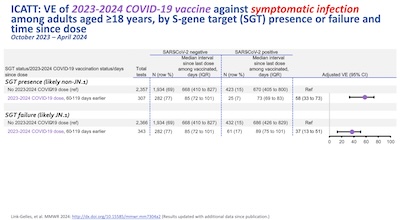
- Ok, they’re different, but do they require a different vaccine? The last plot shows the vaccine efficacy vs non-JN.1 and JN.1 variants. The change to JN.1 causes a loss of 20% of efficacy; among those older than 50 this is enough to wipe out the immunity grant. So, conclusion: yes, we need an updated vaccine!
Natalie Thornburg, CDC: Current epidemiology & genomics
 Next up was Natalie Thornburg, also of the CDC, on the current epidemiology and genomics
of SARS-CoV2. [10]
Next up was Natalie Thornburg, also of the CDC, on the current epidemiology and genomics
of SARS-CoV2. [10]
Basically: where is it, and what variants are out there with which we have to deal? It’s always better to know your opponent’s presence and capabilities, so your defense and counterattack can be more effective!
There were 7 slide in her presentation that stood out to me, documenting the continuing rates of infection, and the importance of the JN.1 lineage:
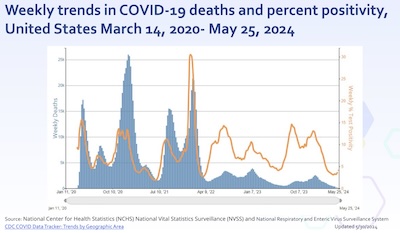
- We have 4 years of COVID-19 history. Thornburg first examines this, looking at about
4.4 years of weekly death rates and weekly test positivity rates. (Though I don’t know
exactly where the test positivity rates come from, given that people almost never
report home test results. There’s something called the National Respiratory and Enteric
Virus Surveillance System, about which I know nothing but which looks interesting.) The
lesson is clear:
- Before about 2022-Apr, death rates and positivity rates were highly correlated. People got COVID-19 with some probability, and they died with some additional probability.
- But after 2022-Apr, they decoupled somewhat to the extent that while they are still clearly related, the death rates went down by a factor of 2 or 3. This is because of population-wide immunity: people either got vaccinated (the smart way) or they rolled the dice and happened to survive COVID-19 (the stupid way). Either way, there was more immunity and less death.
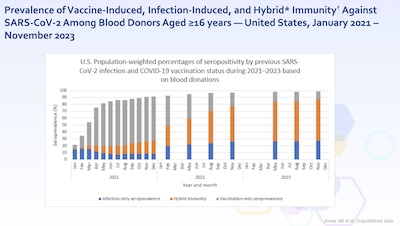
- Next we look at the seroprevalence of various immunity markers in the US population,
over time.
- Some immunity comes from vaccination, some from infection, and some from both.
- This graph tells us 2 things:
- At this point, 90% or more of the population has some kind of exposure like this.
- The vaccine-only group is shrinking, as they gradually get infected. However,
because they’re vaccinated, it’s usually a low-consequence infection. There are
“breakthrough” infections, but vaccines still keep you out of the hospital.
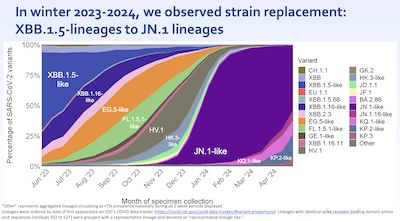
- The lineages active winter 2023-2024 are a rather sad tale. In summer 2023, the FDA
approved a monovalent vaccine based on XBB.1.5. This is what subsequently happened:
- XBB.1.5 quickly died out, being replaced by mostly JN.1. JN.1 is not a descendant of XBB.1.5, but rather a de novo strain from much higher up in the phylogentic tree of SARS-CoV2 strains. Thus, cross-immunity from an XBB.1.5 vaccine was limited.
- It’s a story of being behind the virus, always deciding on a strain and then letting the virus mutate away from it. We have to move faster, and make more sure of the relevance of our vaccinations.
- Right now, the big bears in the woods are JN.1, KP.2, and KP.3 (q.v.). Fortunately, they’re
all closely related (q.v.), so a vaccine against JN.1 will work against the
others. But who knows what will happen this winter?!
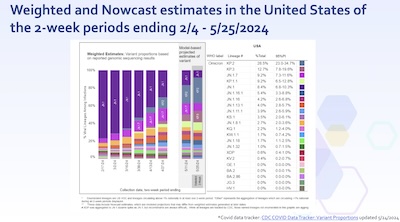
- The ‘nowcast’ confirms this. Since February, JN.1 has been dominant. But now t is
being supplanted, by KP.2, KP.3, and JN.1.7. Have a look at the variant names in the
table on the right: every name there with prevalence > 1% is in the JN.1 family, as
you’ll see on the lineage diagram that comes next. Clear Lesson: JN.1 and its
descendants are dominant now. There’s nothing on the horizon now that looks more
important, though it’s impossible to know what will happen by winter. Still, if you
have to bet – and, alas, you do – then betting your vaccine strain on the
JN.1 family is the best bet known for now.
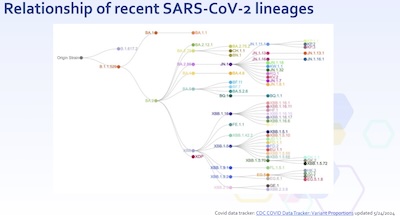
- How are all these variants related? We have a statistical technique that can take the genetic sequence and arrange it into a phylogenetic tree, as shown here, based on the similarities and the mutations. It’s not perfect – people argue all the time about the relevance of “lumpers” and “splitters” – but it’s good enough for what we want to know.
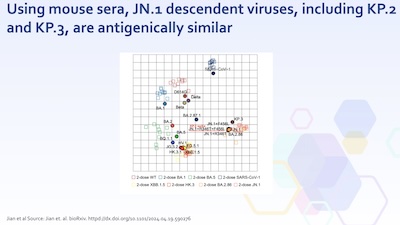
- If we move beyond sequence similarity/difference and look at how an actual immune system responds, then we have this slide on “antigentic distance” (in mice). Now, there’s a lot going on here: multidimensional immune readouts represented in 2 dimensions with a variety of techniques (multidimensional scaling, principal components, singular value decompositions, tSNE clusters, …). But suffice to say that “close in this plane” means “close in terms of how mouse immune systems respond”.
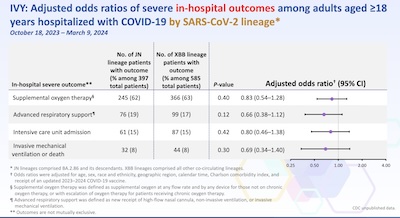
- Finally, we have to ask if this is really worth it. If JN.1, KP.2, and KP.3 were remarkably milder than the previous strains, we might not bother. That’s the point here, looking at odds ratios for the need of various hospital interventions like oxygen, between XBB infections and JN infections. Note that all the odds ratios are not statistically significantly different from 1, i.e., the severity is about the same. So: yes, this is worth it.
David Wentworth, WHO: Technical Advisory Group on COVID-19 Vaccine Composition
 David Wentworth of the World Health Organization spoke next. He’s part of something
called the Technical Advisory Group on COVID-19 Vaccine Composition, or TAG-CO-VAC. Silly
abbreviations aside, this seems exactly on-point for the meeting and was a perspective on
the world outside the US that we too frequently ignore. [11]
David Wentworth of the World Health Organization spoke next. He’s part of something
called the Technical Advisory Group on COVID-19 Vaccine Composition, or TAG-CO-VAC. Silly
abbreviations aside, this seems exactly on-point for the meeting and was a perspective on
the world outside the US that we too frequently ignore. [11]
Alas, your humble Weekend Editor didn’t get a great deal out of this talk, beyond the fact that they recommended a monovalent JN.1 composition for the next vaccine, which is pretty much where everybody else went anyway. They looked at a fair amount of data, some even unpublished, on genetics, antigenic characterization, immunogenicity of neutralizing antibodies, vaccine efficacy of current vaccines against XBB and JN lineages, and so on. So it’s not an idle recommendation, I just couldn’t follow it all that well.
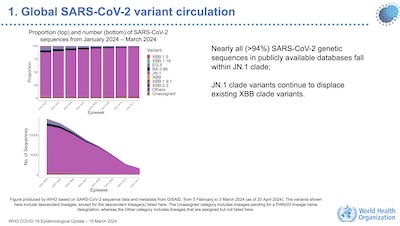 He did present important information showing that nearly all the viral sequences observed
in infected patients (selected how?) across the world are either JN.1 or within the JN.1
clade.
He did present important information showing that nearly all the viral sequences observed
in infected patients (selected how?) across the world are either JN.1 or within the JN.1
clade.
The rest was data similar to that presented above, all of which indicated that XBB.1.5 immunization had some cross-immunity with JN.1, but was not ideal.
So basically all the evidence so far points to JN.1 as the root of the clade for which we want a vaccine. I’d think a multivalent vaccine with JN.1, KP.2, and KP.3 would be ideal, but it appears people preferred just JN.1.
Frances Priddy & Darin Edwards, Moderna: Vaccines Update
 Next was the Moderna presentation. [12] It seems they’ve
developed at-risk vaccines based on both JN.1 and KP.2, which is in the JN.1 lineage but
seems to be overtaking JN.1 now. We can only applaud this effort to get ahead of the
situation and vaccinate against the VoC that’s growing.
Next was the Moderna presentation. [12] It seems they’ve
developed at-risk vaccines based on both JN.1 and KP.2, which is in the JN.1 lineage but
seems to be overtaking JN.1 now. We can only applaud this effort to get ahead of the
situation and vaccinate against the VoC that’s growing.
There was a nice review of the safety of the current XBB.1.5 vaccine (summary: very safe), and cross-neutralization against the new strains (summary: ok, not great).
Then they confirmed what everybody else said, namely that JN.1 is the Big Bad for today, and its lineage (KP.2, KP.3) will probably be the Big Bad for this coming winter.
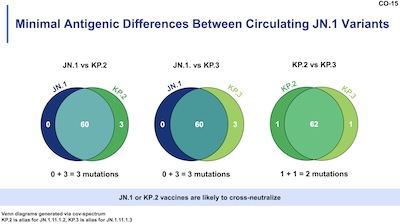 I’ll skip over their murine neutralizing antibody experiments (summary: better antibody
response with new vaccine vs new virus), and simply note that the JN.1 lineage variants
are more similar than we sometimes think. This slide shows Venn diagrams of the
overlapping mutations. Note the very, very small counts unique to each strain (0, 1, 3); these are
really small variations on a theme.
I’ll skip over their murine neutralizing antibody experiments (summary: better antibody
response with new vaccine vs new virus), and simply note that the JN.1 lineage variants
are more similar than we sometimes think. This slide shows Venn diagrams of the
overlapping mutations. Note the very, very small counts unique to each strain (0, 1, 3); these are
really small variations on a theme.
The conclusion from this sequence data is that a JN.1 vaccine should be effective vs KP.2 and KP.3. The immunologic data above confirmed this in vivo in mice, so it’s pretty believable at this point.
They’re prepared to make either a JN.1 or a KP.2 vaccine, whichever gets licensed, for the market starting 2024-Aug. That’s… very good news.
Kayvon Modjarrad, Pfizer/BioNTech: Preclinical & Clinical Supportive Data
 Next was the Pfizer/BioNTech presentation. [13] Like
everybody else, Modjarrad wanted to talk about (a) epidemiology, (2) performance of the
existing XBB.1.5 vaccine, and (iii) preclinical data on their JN.1 vaccine.
Next was the Pfizer/BioNTech presentation. [13] Like
everybody else, Modjarrad wanted to talk about (a) epidemiology, (2) performance of the
existing XBB.1.5 vaccine, and (iii) preclinical data on their JN.1 vaccine.
There’s lots of murine data, but the summary is about what you’d expect: the XBB vaccine works well against XBB.1.5 and less well vs JN lineage; the JN.1 and KP.2 vaccine works better against the JN lineage.
Like Moderna, Pfizer/BioNTech is prepared to supply either JN.1 or KP.2 vaccines upon approval. Again, good news.
Robert Walker, Novavax: Data in Support of Vaccine Update
 The final commercial presentation was from Novavax. [14]
In a way, we kinda have to admire them, for still being in the game with a protein vaccine
despite the general superiority of the mRNA vaccines.
The final commercial presentation was from Novavax. [14]
In a way, we kinda have to admire them, for still being in the game with a protein vaccine
despite the general superiority of the mRNA vaccines.
But we also have to ask why they’re still doing this. Apparently the point of having a protein vaccine, comparable or slightly less effective than mRNA, is to appeal to people who fear the mRNA vaccines. Given the data, that fear must be regarded as superstitious at this point. But it’s a real fear, and if the only way we can vaccinate them is this way, then… I guess we do it this way.
They report the same stuff: epidemiology, XBB vaccine performance, and preclinical data on a JN.1 vaccine. Most of that says the same thing as everybody else, so we’ll concentrate on their novel contributions with their vaccine.
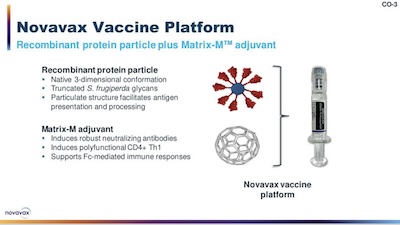 Their vaccine is a protein-based vaccine. They’ve taken an insect protein (S
frugiperda, the fall armyworm moth) and decorated it with multiple copies of the
SARS-CoV2 spike protein. There’s also what’s called an adjuvant, which is basically
something designed to drive your immune system nuts so it reacts more strongly.
Their vaccine is a protein-based vaccine. They’ve taken an insect protein (S
frugiperda, the fall armyworm moth) and decorated it with multiple copies of the
SARS-CoV2 spike protein. There’s also what’s called an adjuvant, which is basically
something designed to drive your immune system nuts so it reacts more strongly.
I gotta say, if people are scared of mRNA, I don’t get why they’re not scared of a fall armyworm protein decorated with viral proteins. Clearly the anti-vax propaganda is very specific in its effect on them!
The vaccine shows antibody responses about where you might expect. I didn’t see a head-to-head comparison with either mRNA vaccine, probably because they’re not available to Novavax to use in their labs.
Novavax is prepared to manufacture a JN.1 vaccine for shipment to US warehouses in 2024-Aug, though no word about international availability. The slower speed and difficulty of retargeting the protein vaccine platforms means they probably can’t supply internationally, and can’t cope with a target change away from JN.1 if the VRBPAC were to mandate that.
Fortunately for Novavax, it looks at this point as though the stars are aligning toward JN.1.
Jerry Weir, FDA: FDA Recommendations on Vaccine Composition
 The final presentation was from the FDA itself. [15]
The final presentation was from the FDA itself. [15]
They start out explaining how we got here in previous VRBPAC meetings. This is important, because it illustrates the need to get out ahead of the viral variants. Being too conservative means giving a vaccine in the fall that is marginal instead of extremely good. Particularly for elders, this is life and death, and now the whole VRBPAC understands that. (I hope.)
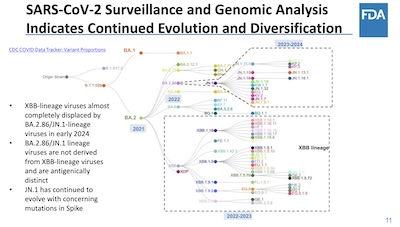 Epidemiologically, they show the same information as everybody else: the JN lineage is
pretty distant from the XBB lineage, and it’s more or less completely dominant now in the
US and in much of the world. KP.2 and KP.3 are catching up, but as we saw in the Venn
diagrams above, these are minor elaborations on the theme of JN. I particularly liked the
way they elaborated the phylogenetic tree of lineages, showing the XBB and JN lineages in
separate dashed boxes: it’s illustration rather than statistics, but it makes the point.
Epidemiologically, they show the same information as everybody else: the JN lineage is
pretty distant from the XBB lineage, and it’s more or less completely dominant now in the
US and in much of the world. KP.2 and KP.3 are catching up, but as we saw in the Venn
diagrams above, these are minor elaborations on the theme of JN. I particularly liked the
way they elaborated the phylogenetic tree of lineages, showing the XBB and JN lineages in
separate dashed boxes: it’s illustration rather than statistics, but it makes the point.
They confirm what everybody else says: JN.1/KP.2/KP.3 are dominant, XBB vaccines are only sorta ok-ish against them, and JN.1 vaccines will be better.
Discussion & Voting
Discussion
 Interestingly, the chair Arnold Monto, was asked about multivalent formulations, and said
“I’m gonna rule that out of order”, because the voting question was already fixed. Seems
unfortunate, since multivalent mRNA vaccines are not much harder than monovalent. Really
all we have to show is that they don’t cause stronger immune reactions? Hmpf.
Interestingly, the chair Arnold Monto, was asked about multivalent formulations, and said
“I’m gonna rule that out of order”, because the voting question was already fixed. Seems
unfortunate, since multivalent mRNA vaccines are not much harder than monovalent. Really
all we have to show is that they don’t cause stronger immune reactions? Hmpf.
This is a 2-stage decision: first decide the lineage (JN.1 or not) and then the specific viruses. Who decides the specific virus, since the manufacturers differ right now in their prior choices? Does the CDC do that?
Looks like it was indeed a slam-dunk in favor of JN.1 lineage, as I predicted. (Chair Monto: “The committee is unusually silent and in agreement.”)
Voting
There are 8 voting members + 8 temporary voting members, for 16 in total. Polled the committee, everybody gets 1 minute to pontificate, an opportunity to change minds at the end, and then after that votes are locked in.
Oddly, they went silent on Zoom and escorted all non-voting members out of the room during voting. I completely fail to see what a secret ballot adds here, unless somebody was afraid of being intimidated in public? Weird.
Results: Called by name (defeating vote secrecy?!) 16 yes to 0 no to 0 abstain.
More Discussion
Arnold Monto suggests they discuss here which strain of the JN.1 variants should go into the monovalent vaccine:
- Whatever we recommend today probably won’t be the one circulating in winter, but the cross-protection is strong. So JN.1 is a strong candidate on the basis that it’s “the trunk of the tree” and not some random branch. *** SARS CoV2 lineage figure to show “trunk of the tree” meaning
- Given Novavax, the only non-mRNA vaccine, is JN.1 there will be problems if we switch because Novavax can’t move fast. That may cause equity problems for those reluctant to accept an mRNA vaccine.
- Having both a protein-based and an mRNA-based vaccine at the same time is probably good. But let’s not get into this position in the future, where we’re constrained by the slowest manufacturing type.
- Don’t know if most prevalent variants will mutate back toward JN.1, or away from it.
And if away from it, we don’t know what the cross-immunity will be. Also, KP.2 and KP.3
appear to be evolving in different directions away from JN.1, so it’s not even just one
choice.
- Weekend Editorial: Seems to me that’s a good argument for a multivalent formulation!
- A couple others agree, e.g., JN.1 and KP.2 or KP.3. But we can’t do that, because it will make Novavax different and introduce complexity in getting physician and patient compliance.
- Monto: previously when we did a multivalent formulation, we had very different strains, but here they’re pretty similar.
- The main adverse events are anaphylaxis and myocarditis, but those are so rare you
really need large studies to see them with any statistical significance.
- Weekend Editorial: Because the effect size is so small!
- KP.2 is probably too specific, and JN.1 is a safer bet?
- Pragmatism: “If we make a good recommendation that people are simply not going to follow, that’s harmful to vaccination in general.”
Adjourned at 3:26PM EDT, endorsing a monovalent JN.1 formulation for 2024-2025.
The Weekend Conclusion
They got 1 “hmpf” + 1 “weird” from me today. A couple of other oddities:
- JN.1 pronounced as “Jane-1”, like “your evil sister Jane”
- “FLiRT mutations” is of course always fun:
- These variants, JN.1 and descendants, have mutations F456L, V1104L and R346T.
- F456L means at position 456 on the spike protein, an amino acid phenylalanine (F) was changed to leucine (L).
- V1104L, similarly changed at position 1104 a valine (V) to leucine (L).
- R346T means at position 346, an arginine (R) was changed to a threonine (T).
- Somebody looked at F456L and R346T and decided the abbreviation “FLiRT” was a mnemonic (though it left out all the other mutations, e.g., V1104L and a host of others).
- These variants, JN.1 and descendants, have mutations F456L, V1104L and R346T.
It looks like they converged on a monovalent JN.1 vaccine, rejecting multivalent alternatives as out of scope and possibly because cross-immunity from JN.1 would be sufficient.
Clear enough. Good enough.
When it’s available in the fall, the Weekend Editrix and I will fire up the Weekend Zeppelin for a trip to go get it. Probably along with a flu vaccination. Possibly with an RSV vaccination, though I don’t know if those are annual or not.
(Ceterum censeo, Trump incarcerandam esse.)
Addendum 2024-Jun-13: FDA Overrides VRBPAC, Specifies KP.2 Strain
Usually, the FDA takes the advice of its advisory committees. After all, why convene a panel of subject-matter experts and then not take their advice?
 Usually, as Derek Lowe points out at In the Pipeline [16],
this is when the ad board votes “no”, and the FDA insists on “yes”, presumably because
they need to be seen Doing Something About the Problem. Aducanumab for Alzheimer’s
appears to be a recent example. The reverse, where the ad board says “no” but the FDA
insists on “yes” is so rare I can’t think of a recent example.
Usually, as Derek Lowe points out at In the Pipeline [16],
this is when the ad board votes “no”, and the FDA insists on “yes”, presumably because
they need to be seen Doing Something About the Problem. Aducanumab for Alzheimer’s
appears to be a recent example. The reverse, where the ad board says “no” but the FDA
insists on “yes” is so rare I can’t think of a recent example.
The FDA/advisory committee relationship is fraught with tension. And that’s as it should be: if there were no tension, everyone would be in agreement, and there would be no need for advice.
Today we have a weird case.
The VRBPAC approved using the JN.1 strain in the fall COVID-19 vaccine, after some discussion about KP.2/3 variants being deemed genetically and immunologically so close it would make no difference. The only thing the VRBPAC could vote on was “JN.1, yes or no”. Voting for KP.2 or KP.3 was not permitted. (See the Voting Question above, and the chair ruling multivalent discussion “out of order”.)
 Today the FDA decided to go with KP.2 anyway [17],
despite not permitting its advisory committee to say that. My bet is there was
considerable behind-the-scenes persuasion putting pressure on them to allow KP.2, since
the VRBPAC was effectively muzzled in this regard.
Today the FDA decided to go with KP.2 anyway [17],
despite not permitting its advisory committee to say that. My bet is there was
considerable behind-the-scenes persuasion putting pressure on them to allow KP.2, since
the VRBPAC was effectively muzzled in this regard.
So… is this a good thing or a bad thing?
First, it’s just a thing: as we pointed out above, occasional friction with an ad board is a healthy sign, as long as it’s not being overridden all the time.
Second, it’s (likely) a small thing: see the evidence above that there are very small antigenic mutations between JN.1 and KP.2/3 and the immunologic response in mice vaccinated against JN.1 was still pretty good.
Third, it’s a mixed, small thing:
- On the negative side, Novavax will still have to go with JN.1. They’re a protein vaccine, and we can’t pivot those as quickly as mRNA vaccines. This is inevitable, and is in some sense a good thing since it highlights a good feature of the mRNA vaccines.
-
On the positive side, there’s a slight improvement in response to KP.2/3, and there may be improved response to whatever likely descendant of KP.2/3 is cursing us in the fall.
On the whole, I’d have preferred to have had the FDA give the VRBPAC a voting question flexible enough to include KP.2/3 and multivalent formulations, instead of “JN.1: yes or no”. But as all the existentialists say, “Nevertheless, here we are.” This sounds to me like it’s somewhere from “sort of ok” to “pretty good”, and I’m happy with that.
Fourth, it’s an important, mixed, small thing:
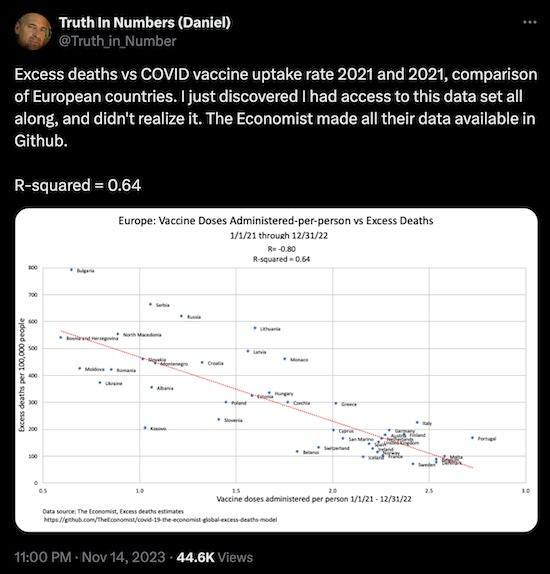
- A statistician looked at some data on European death rates and vaccination rates, collected by The Economist and placed on GitHub for public review.
- The vertical axis is “excess deaths”, i.e., death rates in 2021 and 2022 over and above what would be expected in a non-pandemic year. These are deaths reasonably attributable to COVID-19.
- The horizontal axis is the number of vaccine doses per person. Higher is better, i.e., up to date on immunizations. Only in a country with functional electronic medical records is this possible; it cannot be done in the US because of our crippled healthcare systems.
- The regression line shows an $R^2 \sim 0.64$, i.e., the number of vaccine doses explains about 64% of the variance in the excess deaths. And the variance is a decrease in excess death rates with more vaccination and boosting.
Conclusion: Once again, more vaccination means less death.
If the FDA’s switch to the now-latest-and-greatest KP.2 strain for the fall causes even slightly more uptake of the booster by the public, then fewer people will die.
You should live, and not die (as it says in a very old book).
Notes & References
1: FDA Staff, “Advisory Committee Calendar”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
2: FDA Staff, “Vaccines and Related Biological Products Advisory Committee June 5, 2024 Meeting Announcement”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
3: FDA Staff, “185th Meeting of Vaccines and Related Biological Products Advisory Committee”, YouTube, viewed live 2024-Jun-05. ↩
4: FDA Staff, “185th Vaccines and Related Biological Products Advisory Committee Meeting Roster”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
5: FDA Staff, “Vaccines and Related Biological Products Advisory Committee Meeting Waivers for Conflicts of Interest”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
6: FDA Staff, “185th Meeting of the Vaccines and Related Biological Products Advisory Committee AGENDA”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
7: FDA Staff, “FDA Briefing Document Vaccines and Related Biological Products Advisory Committee Meeting June 5, 2024: Selection of the 2024-2025 Formula for COVID-19 vaccines”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
8: JP Weir, “Selection of the 2024-2025 Formula for COVID-19 Vaccines - Introduction”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
9: R Link-Gelles, “Effectiveness of COVID-19 (2023-2024 Formula) Vaccines”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
10: NJ Thornburg, “Update on Current Epidemiology of COVID-19 and SARS-CoV-2 genomics”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
11: D Wentworth, “Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) Statement on COVID-19 vaccine antigen composition”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
12: F Priddy & D Edwards, “Moderna COVID-19 Vaccines Update”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
13: K Modjarrad, “2024-2025 COVID-19 Vaccine Formula: Pfizer/BioNTech Clinical and Preclinical Supportive Data”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
14: R Walker, “Novavax Data in Support of 2024-2025 Vaccine Update”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
15: JP Weir, “FDA Considerations and Recommendations for the 2024-2025 COVID-19 Vaccine Formula Composition”, US Food & Drug Administration web site, downloaded 2024-Jun-05. ↩
16: D Lowe, “Those Darn FDA Advisory Committees”, In the Pipeline blog at Science, 2024-Jun-17. ↩
17: FDA Staff, “Updated COVID-19 Vaccines for Use in the United States Beginning in Fall 2024, FDA Updates Advice to Manufacturers of COVID-19 Vaccines (2024-2025 Formula): If Feasible Use KP.2 Strain of JN.1-Lineage”, US Food & Drug Administration web site, downloaded 2024-Jun-13. ↩
Gestae Commentaria
Comments for this post are closed pending repair of the comment system, but the Email/Twitter/Mastodon icons at page-top always work.